CORTUCIN-N® – Betamethasone Valerate BP + Neomycin Sulphate BP
COMPOSITION
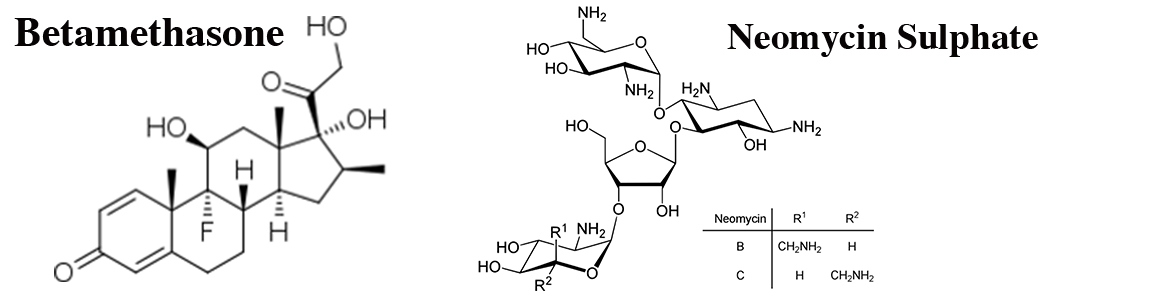
Each gram cream contains Betamethasone Valerate BP 1 mg & Neomycin Sulfate BP 5 mg.
PHARMACOLOGY
Betamethasone Valerate is very active fluorinated corticosteroid for topical use. At normal dosage, even often prolong use, it does not depress the suprarenal glands and one may consider its systemic activity as practically nil. Neomycin Sulfate is a broad spectrum, bactericidal antibiotic effective against the majority of bacteria commonly associated with skin infections.
INDICATION
Indicated for the treatment of the following conditions where secondary bacterial infection is present, suspected, or likely to occur; eczema in adults and children (aged 2 years and over), including atopic and discoid eczemas; prurigo nodularis; psoriasis (excluding widespread plaque psoriasis); neurodermatoses including lichen simplex and lichen planus; seborrheic dermatitis; contact sensitivity reactions; insect bite reactions; and anal and genital intertrigo.
DOSAGE AND ADMINISTRATION
Apply sparingly to the affected area 2 to 3 times daily until an improvement occurs. In children, course should be limited to 5 days.
SIDE EFFECT
Burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae and miliaria may be reported.
PRECAUTION
Long-term continuous topical therapy should be avoided where possible, particularly in infants and children. If applied to the eyelids, care is needed to ensure that the preparation does not enter the eye, as glaucoma might occur.
CONTRAINDICATION
This preparation is contraindicated to the patients who are hypersensitive to any of its components. It should not be used for the treatment of otitis externa when the eardrum is perforated. It is contraindicated in skin lesions caused by infection with viruses and fungi.
DRUG INTERACTION
Possible cumulative toxicity should be considered when Betamethasone Valerate-Neomycin Sulphate is applied topically in concurrent use of CYP3A4 inhibitors (Clarithromycin, Erythromycin, Diltiazem, Itraconazole, Ketoconazole, Ritonavir, Verapamil etc), Neuromuscular blocking agents (Atracurium, Cisatracurium, Nimbex etc) and in systemic aminoglycoside therapy. Pseudomembranous colitis has been reported with the use of antibiotics and may range in severity from mild to life-threatening.
USE IN PREGNANCY AND LACTATION
There are no adequate and well controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids and should not be used extensively for a prolonged period. Use of Betamethasone Valerate-Neomycin Sulphate is not recommended in lactation.
STORAGE
Store at or below 30° C. Do not freeze. Keep out of the reach of children.
PACKAGING
CORTUCIN-N® Cream: Each tube contains 5 gm cream.