E-HEXIN ®– Bromhexine Hydrochloride BP
COMPOSITION
Available in 2 different Dosage forms:
Tablet: Each tablet contains 16mg Bromhexine Hydrochloride BP.
Suspension: Each 5 ml suspension contains Bromhexine equivalent to 4 mg Bromhexine Hydrochloride BP.
PHARMACOLOGY
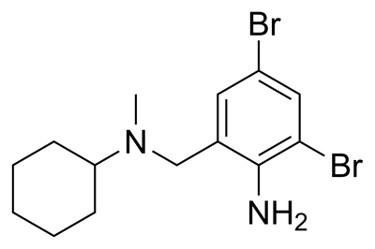
E-Hexin® is a popular expectorant containing Bromhexine Hydrochloride which is a mucolytic/mucokinetic expectorant. Bromhexine Hydrochloride is a synthetic derivative of vasicine, an active ingredient of Adhatoda vasica which acts on bronchi.
It offers expectorant action by the following mechanisms:
* It stimulates the lysosomal enzyme activity.
* It balances mucin synthesis
* It normalizes viscosity and elasticity of mucous by regulating its protein and enzyme content.
* It lowers sialomucin catabolism, thus inhibits degradation of bradykinin, a well-known pyrexial mediator.
* It also increases the membrane permeability whereby the immunoglobulin level in the bronchial secretion rises.
Bromhexine Hydrochloride is absorbed from the gastro-intestinal tract and the peak plasma level is attained after one hour. Bromhexine is highly metabolized in the liver and therefore the bioavailability is low. Most of the dose is excreted in the urine mainly as metabolite. Only a small amount is excreted in the stool.
A special clinical feature of Bromhexine Hydrochloride:
Association of Cephalexin & Amoxycillin with Bromhexine has been proved to effective against both infections and pathological symptoms, particularly, its reduction of fever was rapid, confirming the high antibacterial activity of Antibiotic like Cephalexin and Amoxycillin when administered with Bromhexine which has a superior bronchial bioavailability.
INDICATION
E-Hexin® is a mucolytic/mucokinetic expectorant. This drug has been reported to change the structure of bronchial secretions and to increase the volume and reduce the viscosity of sputum. It is indicated in the cases of
• Acute and Chronic Bronchitis;
• Sinusitis;
• Otitis media;
• Sjogren’s syndrome;
• Post surgical bronchial complications;
DOSAGE AND ADMINISTRATION
Bronchitis:
Adult: 8 to 16 mg three – four times daily
Children (5-12 Years) 8 mg four times daily
Children (below 5 years) 4 mg twice daily
Otitis Media 16 mg twice daily
Lower Respiratory Infections 8 mg every eight hours daily
Along with Antibiotic
Siogren’s Syndrome 8mg every 8 hours daily (normal dose) 16 mg 8 hours daily (in special case)
SIDE EFFECT
There are no serious side effects of Bromhexine HCI. However, gastrointestinal side effects may occur occasionally with bromhexine and a transient rise in serum aminotransferase values has been reported. Other reported adverse effects include headache, vertigo (dizziness), sweating and allergic reactions.
PRECAITION
Since mucolytics may disrupt the gastric mucosal barrier, bromhexine should be used with caution in patients with a history of gastric ulceration. Clearance of bromhexine or its metabolites may be reduced in patients with severe hepatic or renal impairment.
CONTRAINDICATION
Contraindications. Bromhexine is contraindicated for use in patients with known hypersensitivity or idiosyncratic reaction to bromhexine hydrochloride.
DRUG INTERACTION
When given in combination with Bromhexine the concentrations of Chloramphenicol, Tetracycline of Spiramycin in the bronchial secretion are increased and may show symptoms of drug adverse effects of the antibiotics.
USE IN PREGNANCY AND LACTATION
No evidence of ill-effects during pregnancy, but the use of drugs 1st trimester of pregnancy should be observed. Bromhexine HCl is expected to enter breast milk. Therefore, it is not recommended for breastfeeding mothers unless the potential benefits to the patient are weighed against the possible risk to the infant.
STORAGE
Store tablet and suspension at or below 30°C. Protect from light & moisture. Keep out of the reach of children.
PACKAGING
E-HEXIN®: Tablet 16 mg: Each box contains 10 strips of 10 tablets in blister pack.
E-HEXIN®: Suspension: Each bottle contains 100 ml Suspension.