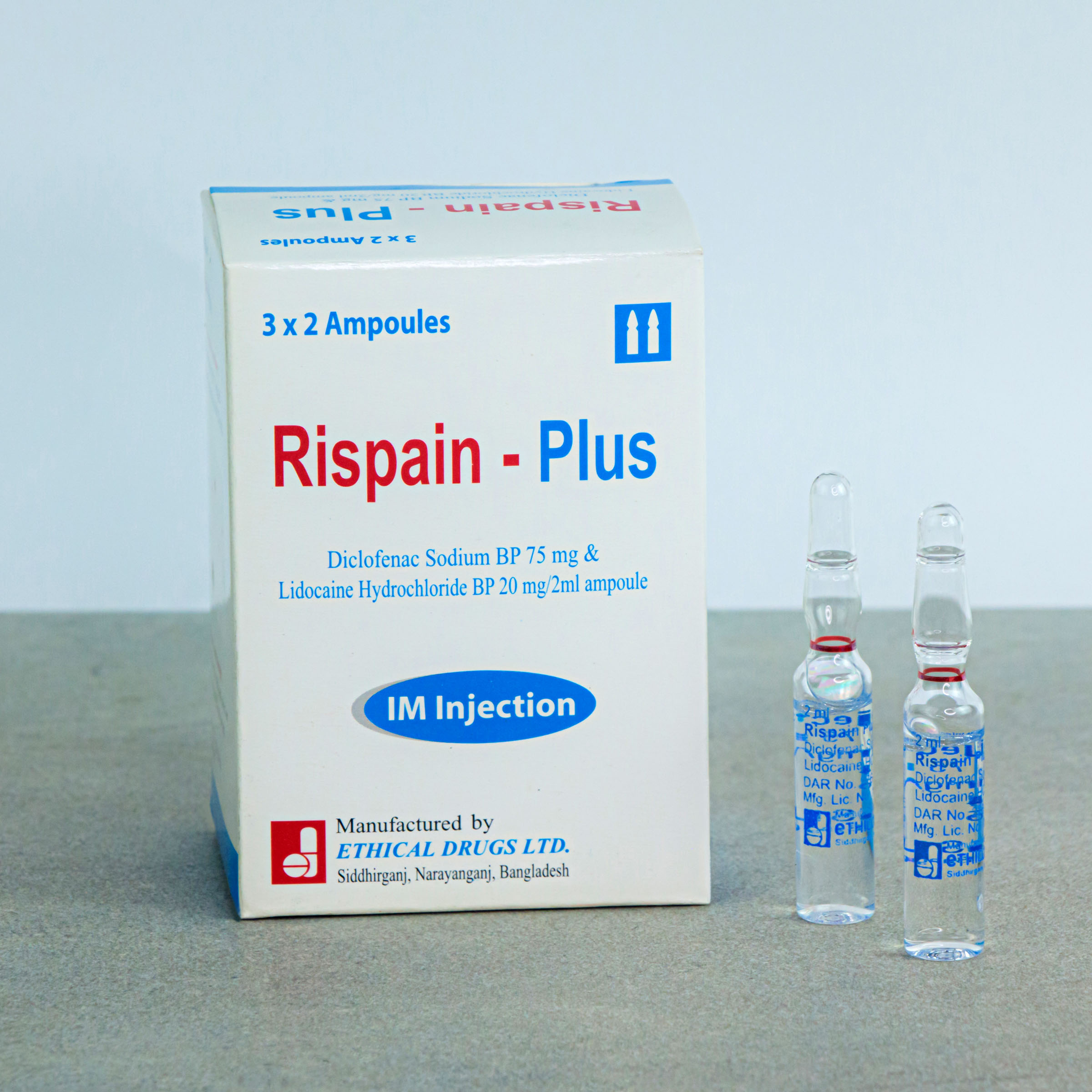
RISPAIN PLUS ®– Diclofenac Sodium + Lidocain Hydrochloride
COMPOSITION
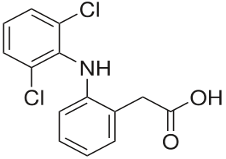
Each 2 ml ampoule of Rispain-Plus Injection contains Diclofenac sodium BP 75 mg and Lidocaine hydrochloride BP 20 mg.
PHARMACOLOGY
Diclofenac is a potent non-steroid anti-inflammatory drug (NSAID) with analgesic and antipyretic properties. Diclofenac inhibits cyclooxygenase activity with a reduction of the tissue production of prostaglandin. Lidocaine (Lignocaine) is effectively absorbed from mucous membranes and is used combined with Diclofenac as a useful surface anaesthetic in concentrations of 2% to 4% to reduce muscular pain at the site of injection.
INDICATION
Rheumatology: Inflammatory and degenerative forms of rheumatism, chronic involutive, polyarthritis. Surgery and Traumatology: Sprain, bruises, dislocations, fractures, soft-tissue injuries, surgical interventions.
Obstetrics and Gynecology: Primary dysmenorrhoea, episiotomy, adnexitis, endometritis, parametritis, salpingitis, and mastitis.
Otorhinolaryngology: As pre-operative medication for the prevention of pain, inflammation, and swelling.
Dentistry: Post-operative and post-traumatic pain, inflammation, and swelling.
Other Indications: For the prevention of pain and treatment of inflammation and swelling of patients operated in the urogenital tract, renal and biliary colic.
DOSAGE AND ADMINISTRATION
In adults:
For post-operative case: One ampoule once daily (in severe cases twice daily) by deep intramuscular injection.
Renal colic: One ampoule once daily by deep intramuscular injection. A further ampoule may be administered after 30 minutes if necessary. The maximum daily dosage of diclofenac sodium is 150 mg & lidocaine hydrochloride is 200 mg.
In Children: For juvenile chronic arthritis: 1-3 mg of diclofenac containing lidocaine/kg body weight in divided doses.
SIDE EFFECT
Effects of diclofenac are mild, rare and transient. It is generally well tolerated. At the starting of the treatment, however, patients may sometimes complain of epigastric pain, eructation, nausea and diarrhoea, or the dizziness or headache. These effects are usually of mild in nature. Peripheral edema and skin reactions, such rash and eczema have also been encountered.
PRECAUTION
In the rare instances where peptic ulceration or gastro-intestinal bleeding occurs in patients under treatment with Diclofenac & Lidocaine Injection. In patient receiving long-term anti-steroid therapy should be monitored as a precautionary measure (renal or hepatic function and counts).
CONTRAINDICATION
Contraindicated in patients with peptic ulcer, hypersensitivity to diclofenac like other non-steroidal anti-inflammatory agents, Rispain-Plus injection is also contraindicated in patients suffering with asthma, urticaria or acute rhinitis.
DRUG INTERACTION
Diclofenac may increase plasma concentration of Lithium, Digoxin, Potassium-sparing diuretics. Combined use with anticoagulants may increase the risk of hemorrhage. Concomitant use of Diclofenac with Cyclosporine & Methotrexate can cause toxicity in patients.
USE IN PREGNANCY AND LACTATION
Diclofenac Sodium- Pregnancy category ‘C’.
Lidocaine Hydrochloride- Pregnancy category ‘B’.
DURING PREGNANCY DICLOFENAC SODIUM AND LIDOCAINE
Hydrochloride should be employed only for compelling reasons. The lowest effective dose should be used. These types of drugs are not recommended during the last trimester of pregnancy. Very insignificant quantity of diclofenac may be detected in breast milk but no undesirable effects on the infant to be expected.
OVER DOSAGE
Symptoms of NSAIDs overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. In rare cases gastrointestinal bleeding, hypertension, acute renal failure, respiratory depression and coma may occur. Also anaphylactic reactions may occur following an overdose of NSAID.
STORAGE
Store at or below 30°C. Protect from light & moisture. Keep out of the reach of children.
PACKING
Each box contains 3 X 2’s ampoules in blister pack.