Ivermec® Vet Injection
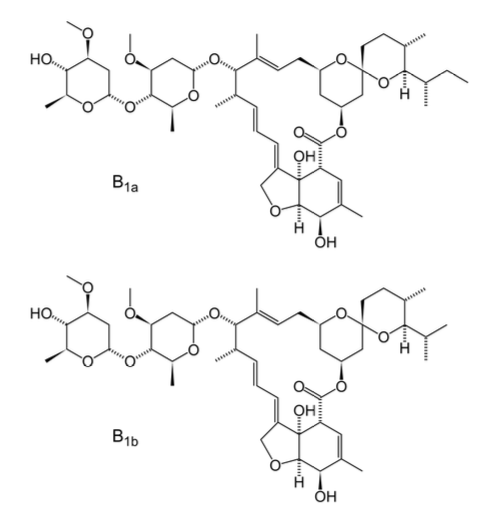
Ivermectin BP
COMPOSITION
Each ml injection contains Ivermectin BP 10 mg.
MODE OF ACTION
Ivermec® Vet Injection (Ivermectin BP 10 mg) Ivermectin is a member of the macrocyclic lactone class of endectocides which have an unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerves and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA). The margin of safety for compounds of this class is attributable to the fact that mammals do not have glutamate-gated chloride channels, the macrocyclic lactones have a low affinity for other mammalian ligand-gated chloride channels and they do not readily cross the blood-brain barrier.
INDICATION
Ivermec® Vet Injection is indicated for the treatment and control of the following internal and external parasites of cattle, buffalo, sheep, goat and horse.
Internal Parasite
Gastrointestinal roundworms: Ostertagia, Haemonchus, Strongylus, Trichostrongylus, Metastrongylus, Strongyloides, Cooperia, Oesophagostomum, Bunostomum, Nematodirus, Mecistocirrus, Toxocara, Trichuris, Oxyuris, Parascaris, Ancylostoma spp. etc.
Lungworms: Dictyocaulus viviparous, Dictyocaulus filaria, Dictyocaulus arnfieldi, Protostrongylus rufescens.
External Parasites
Fly: Hypoderma bovis, H. lineatum, Crysomya bovis
Sucking lice: Linognathus vituli, Haematopinus eurysternus, Solenopotes capillatus.
Biting lice: Damalinia.
Mange mites: Psoroptes ovis, Sarcoptes scabiei var. bovis, Chorioptes, Demodex, Otodectes, Notoedres.
Tick: Boophilus ornithodorus.
Ivermec® Vet Injection is also indicated for treatment and control of humpsore (Stephanofilaria assamensis) in cattle.
DOSAGE AND ADMINISTRATION
Method of administration: Should be injected only by subcutaneous route (under the loose skin in front of or behind the shoulder).
Ruminant : 1 ml/ 50 kg body weight or 0.2 mg/kg body weight and repeat dose after 14 days. In case of humpsore the dose may be repeated after 28 days.
Dog : 0.5ml/ 25 kg body weight or 0.2 mg/kg body weight and repeat dose after 14 days.
In the treatment of mange, the dosage should be as follows-
Otodectic mange: Single injection.
Sarcoptic mange: 2 injections at a 14 days interval.
Demodectic mange and Fleas: 3 injections at a 7 days interval.
Cat : 0.5ml/25 kg body weight or 0.2 mg/kg body weight (Single injection).
Horse : 1 ml/50 kg body weight or 0.2 mg/kg body weight (Single injection) in I/M route.
Or, as per direction of Registered Veterinarian.
CONTRAINDICATION
Animals with a history of hypersensitivity to Ivermectin or to other macrocyclic lactones are contra-indicated for this drug.
SIDE EFFECT
Transitory discomfort has been observed in some cattle following subcutaneous administration. A low incidence of soft tissue swelling at the injection site has been observed. These reactions have disappeared without treatment.
USE IN PREGNANCY AND LACTATION
Ivermec® Vet Injection is considered to be safe for use during pregnancy. Reproductive studies performed in dogs, horses, cattle and swine have not demonstrated any adverse effects to fetuses. Reproductive performance in male animals is also apparently unaltered.
WITHDRAWAL PERIOD
Meat: 21 days, milk: 28 days.
STORAGE
Keep away from light in a cool and dry place.
Keep all the medicines out of reach of children.
PACK SIZE
10 ml and 30 ml injectable vials.