GASPAIN®–Pantoprazole INN
COMPOSITION
20 Tablet: Each enteric coated tablet contains Pantoprazole sodium Sesquihydrate INN equivalent to Pantoprazole 20mg
40 Tablet: Each enteric coated tablet contains Pantoprazole sodium Sesquihydrate INN equivalent to Pantoprazole 40mg
PHARMACOLOGY
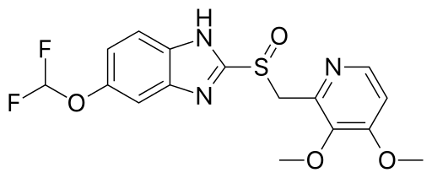
Pantoprazole is chemically a novel substituted benzimidazole derivative, which suppresses the final step in gastric acid production by forming a covalent bond to two sites of the H+, K+ – ATPase enzyme system at the secretory surface of the gastric parietal cell. This leads to inhibition of both basal and stimulated gastric acid secretion irrespective of the stimulus. The binding to the H+, K+ – ATPase results in duration of antisecretory effect that persists longer than 24 hours. Pantoprazole is quantitatively absorbed and bioavailability does not change upon multiple dosing. Pantoprazole is extensively metabolized in the liver. Almost 80% of an oral dose is excreted as metabolites in urine; the remainder is found in feces and originates from biliary secretion.
INDICATION
Peptic ulcer diseases (PUD), Gastro esophageal reflux diseases (GERD), Treatment of ulcer resistant to H2 receptor antagonists (H2RAs), Treatment of ulcers induced by non-steroidal anti-inflammatory drugs (NSAIDs), Gastrointestinal (GI) bleeding from stress or acid peptic diseases, Eradication of Helicobacter pylori (in combination with antibiotics), Zollinger-Ellison syndrome, Prophylaxis for acid aspiration syndrome during induction of anesthesia
DOSAGE AND ADMINISTRATION
Benign gastric ulcer: 40 mg daily in the morning for 4 weeks, continued for further 4 weeks, if not fully healed. Gastro-esophageal reflux disease: 20-40 mg daily in the morning for 4 weeks, continued for further 4 weeks, if not fully healed; maintenance dose is 20 mg daily, which may be increased to 40 mg daily. Duodenal ulcer: 40 mg once daily in the morning for 2 weeks, continued for further 2 weeks if not fully healed. Duodenal ulcer associated with Helicobacter pylori: Pantoprazole is recommended at a dose of 40mg twice daily in association with antimicrobial agents as detailed below: Amoxycillin 1 g and Clarithromycin 500 mg both twice daily for one week, or Clarithromycin 250 mg and Metronidazole 400 mg both twice daily for one week. Prophylaxis of NSAID-associated gastric or duodenal ulcer: 20 mg daily for those require long-term NSAID treatment. Zollinger-Ellison Syndrome: Initially 80mg once daily adjusted according to response (elderly max. 40 mg daily); daily doses above 80 mg given in 2 divided doses.
SIDE EFFECT
Pantoprazole is well tolerated in both short-term and long-term treatment. Headache and diarrhea are the common side effects and rarely included side effects are abdominal pain, flatulence, rash, insomnia and hyperglycemia.
PRECAUTION
Patients should be cautioned that pantoprazole delayed release tablets should not be split, chewed or crushed.
DRUG INTERACTION
There is no interaction with concomitantly administered antacids. No dosage adjustment in needed with combination use of the following drugs: Theophylline, Caffeine, Diazepam, Digoxin, Ethanol, Metoprolol, Nifedipine, Warfarin.
USE IN PREGNANCY AND LACTATION
Studies using animals have not found any risk to fetus. No data are available on administration of Pantoprazole in neonates & children.
STORAGE
Store in a cool, dry place & below 30° C. Protected from light. Keep out of reach of children.
PACKAGING
GASPAIN®: Tablet 20mg: Each Box contains 50 tablet in Alu-Alu blister strips.
GASPAIN®: Tablet 40mg: Each Box contains 50 tablet in Alu-Alu blister strips.