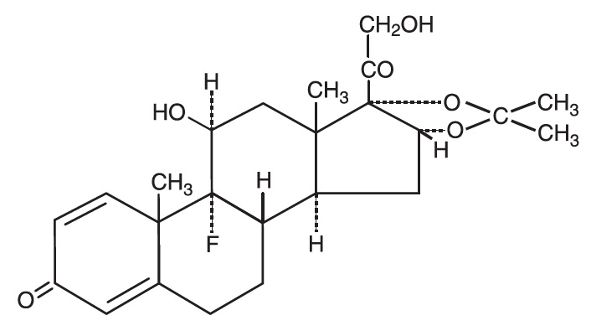
EMICORT®– Triamcinolone Acetonide Injection BP
COMPOSITION
Each 1 ml vial contains Triamcinolone Acetonide BP 40 mg sterile IM/IA injectable suspension.
PHARMACOLOGY
Triamcinolone Acetonide is synthetic glucocorticoid corticosteroids with marked anti-inflammatory actions which also have salt retaining properties, which are used as replacement therapy in adrenocortical deficiency states. So it is primarily used for their potent anti-inflammatory effects in disorder of many organ systems.
INDICATION
Intra-Muscular (IM) – The IM administration of Triamcinolone Acetonide (Sterile Triamcinolone Acetonide Suspension BP) is indicated for systemic corticosteroid therapy in such conditions as allergic diseases, dermatosis, generalized rheumatoid arthritis, neurological disorder, nervous system and hematologic disorder. Intramuscular administration is particularly valuable when oral corticosteroid therapy is not feasible. Intra-Articular (IA)- The Intra-articular administration of Triamcinolone Acetonide injection is indicated for adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in synovitis of osteoarthritis, rheumatoid arthritis, acute and sub-acute bursitis, acute gouty arthritis, epicondylitis, acute nonspecific tenosynovitis and post-traumatic osteoarthritis.
DOSAGE & ADMINISTRATION
The dose of Triamcinolone Acetonide is dependent on the size of the joint to be treated and on the severity of the condition. Doses of 2.5-5 mg (0.0625-0.1250 ml) for smaller joint and 5-15 mg (0.125-0.375 ml) for larger joints usually alleviate the symptoms. Triamcinolone Acetonide 40 mg/ml is available to facilitate administration of larger doses or, as directed by the registered physician.
SIDE EFFECT
Muscle weakness, fatigue, steroid myopathy, loss of muscle mass, osteoporosis, dyspepsia, pancreatitis, impaired wound healing etc.
PRECAUTION
Particular care is required when considering use of systemic corticosteroids in patients with the following conditions and frequent patient monitoring is necessary. Diverticulitis, thrombophlebitis, existing or previous history of severe affective disorders (especially previous steroid psychosis), exanthematous diseases, chronic nephritis, or renal insufficiency, metastatic carcinoma, osteoporosis (post-menopausal females are particularly at risk); in patients with an active peptic ulcer (or a history of peptic ulcer).
CONTRAINDICATION
This is contraindicated in patients with known hypersensitivity to any of the ingredients of the formulation. Moreover, this is also contraindicated in systemic infections unless specific anti-infective therapy is employed. Besides this, administration by intravenous or intrathecal injection is contraindicated.
DRUG INTERACTION
Barbiturates, phenytoin, rifampicin, rifabutin, carbamazepine, primidone and aminoglutethimide may enhance the metabolic clearance of corticosteroids, resulting in decreased therapeutic effects.
USE IN PREGNANCY & LACTATION
Triamcinolone Acetonide is a pregnancy category “C” drug. As with all drugs, Triamcinolone should only be prescribed when the benefits to the mother and child outweigh the risks. Corticosteroids may pass into breast milk; although, no data are available for Triamcinolone.
OVER DOSAGE
Data on human has not been found. Patients experiencing an overdose may develop Cushing’s syndrome. This overdose may be treated with supportive therapy and mifepristone for its ant glucocorticoid activity.
STORAGE
Store at or below 30°C. Protect from light & moisture. Keep out of the reach of children.
Packing: Each box contains 1 x 3 vials of injectable suspension.
PACKAGING:
Each Box contains 1 x 3 vials of injectable suspension