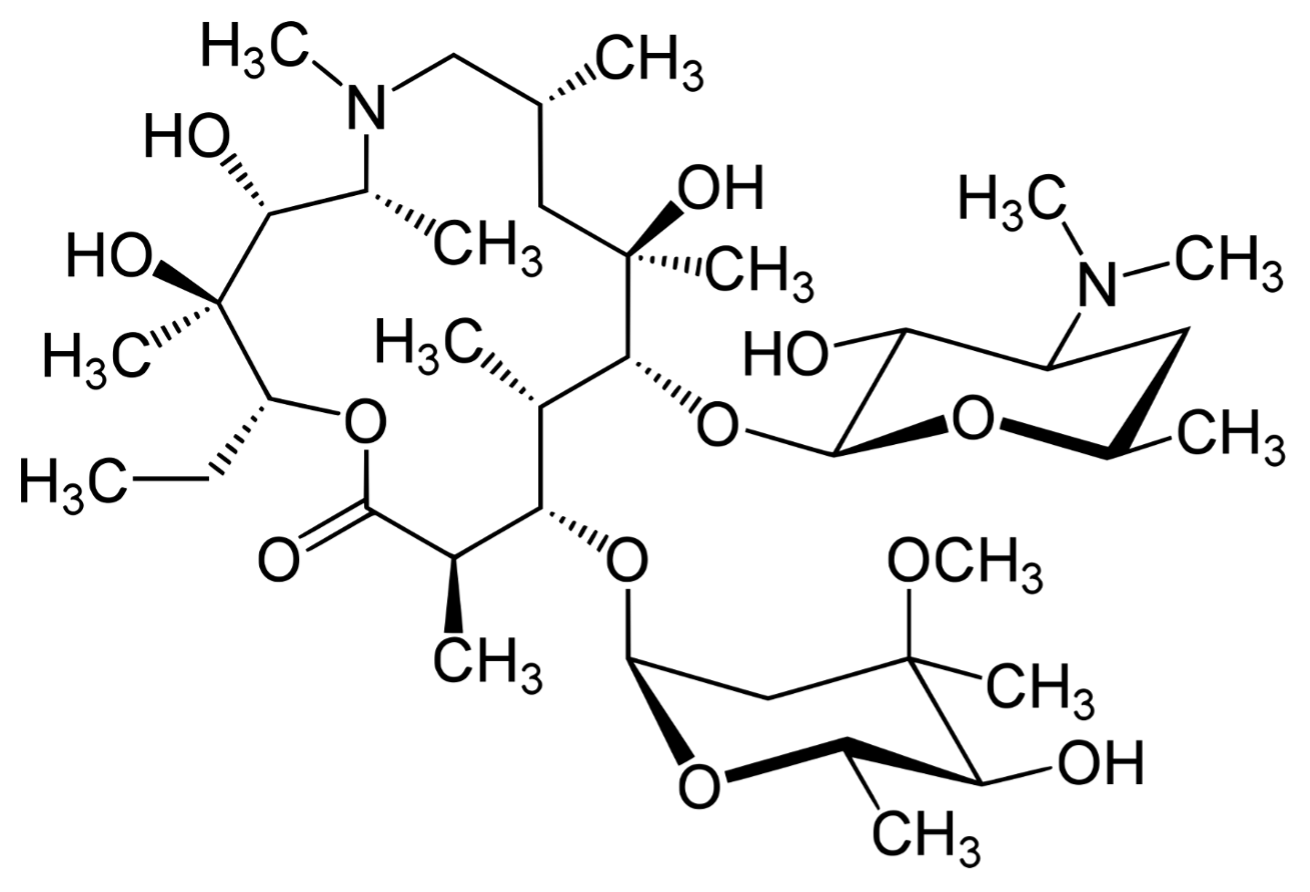
MACLID ® – Azithromycin Dihydrate USP
COMPOSITION
Available in 2 different Dosage forms:
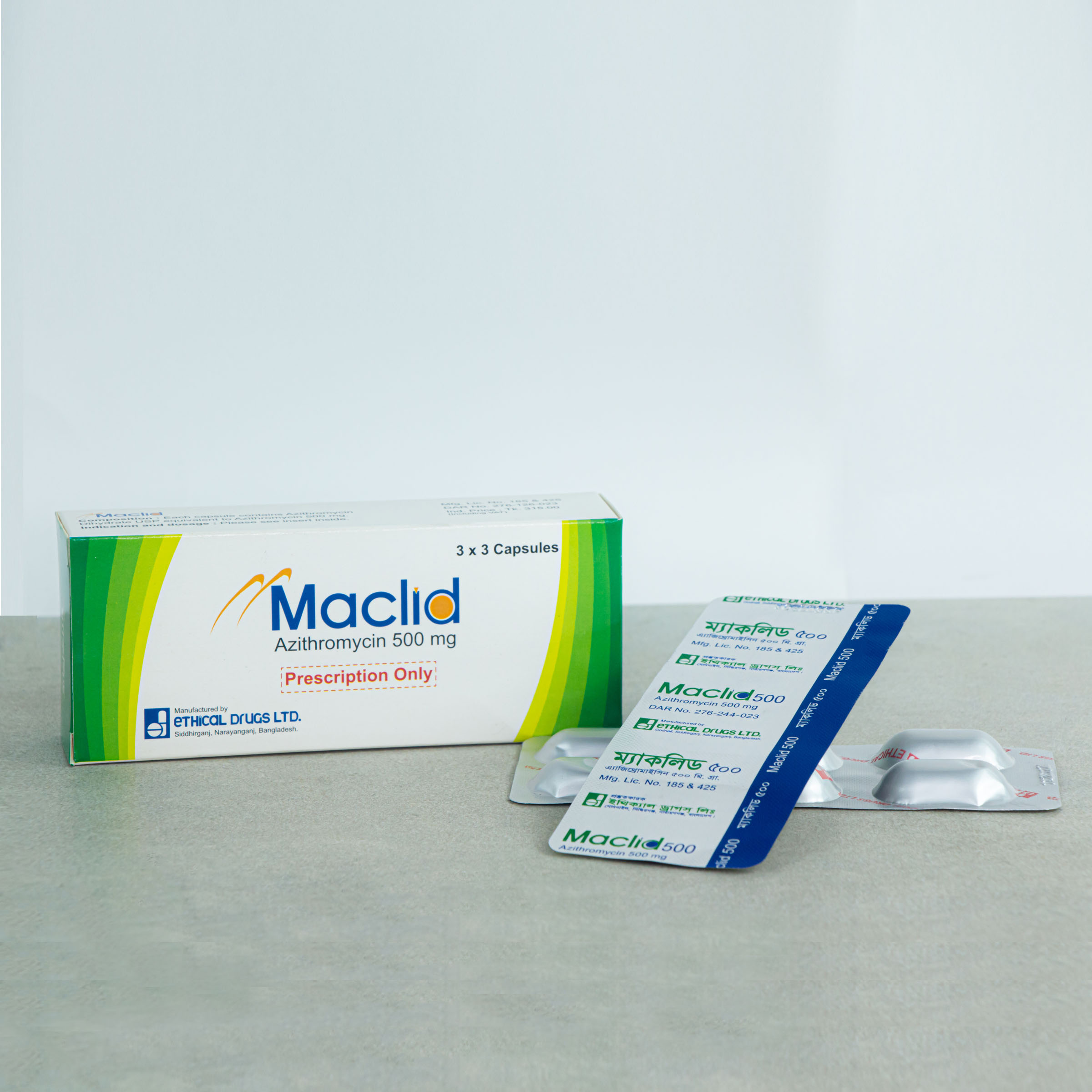
500 mg Capsule: Each capsule contains 500 mg Azithromycin.
250 mg Capsule: Each capsule contains 500 mg Azithromycin.
500 mg Tablet: Each film coated tablet contains 500 mg Azithromycin.
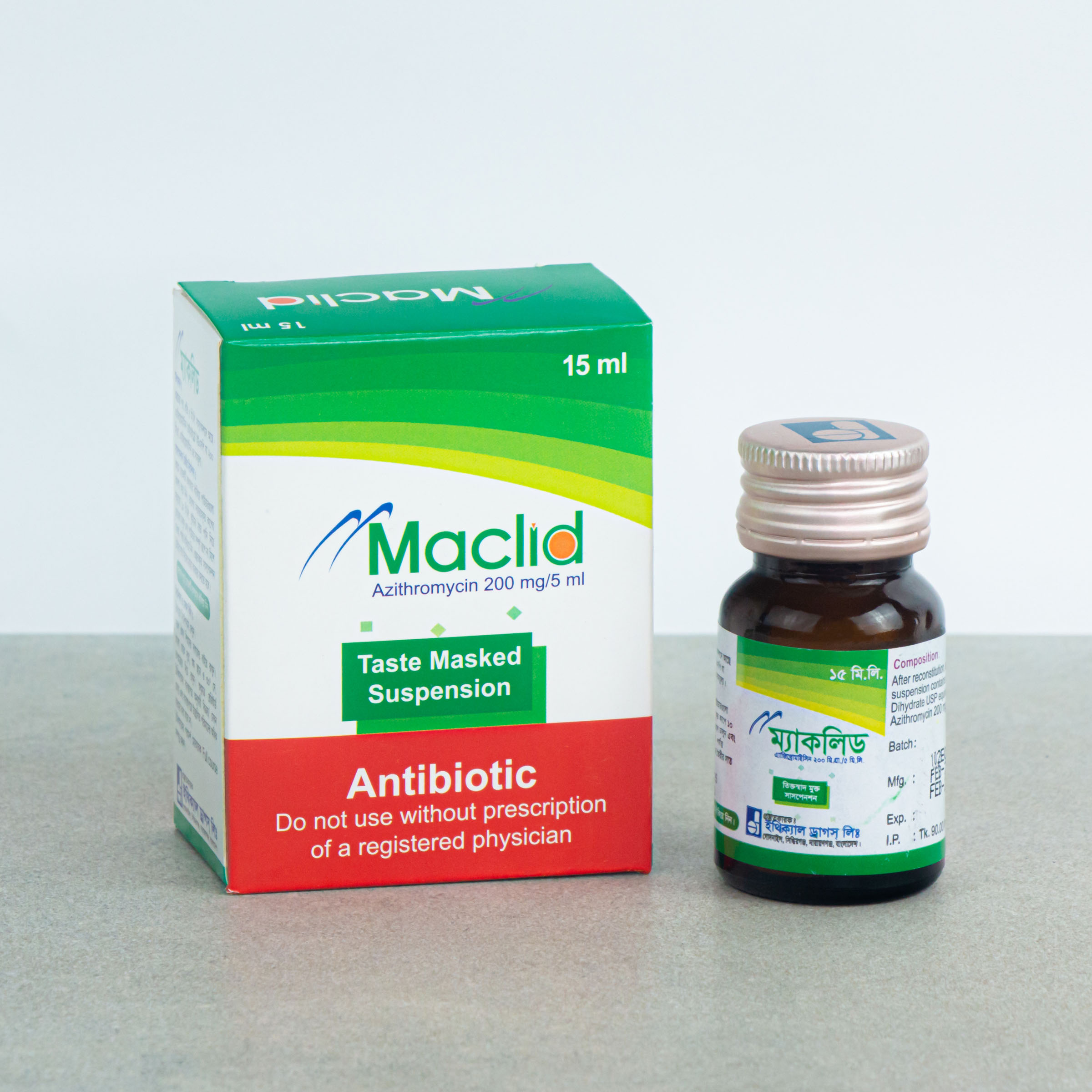
15 ml Powder for Suspension: Each Bottle contain 200 mg of Azithromycin.
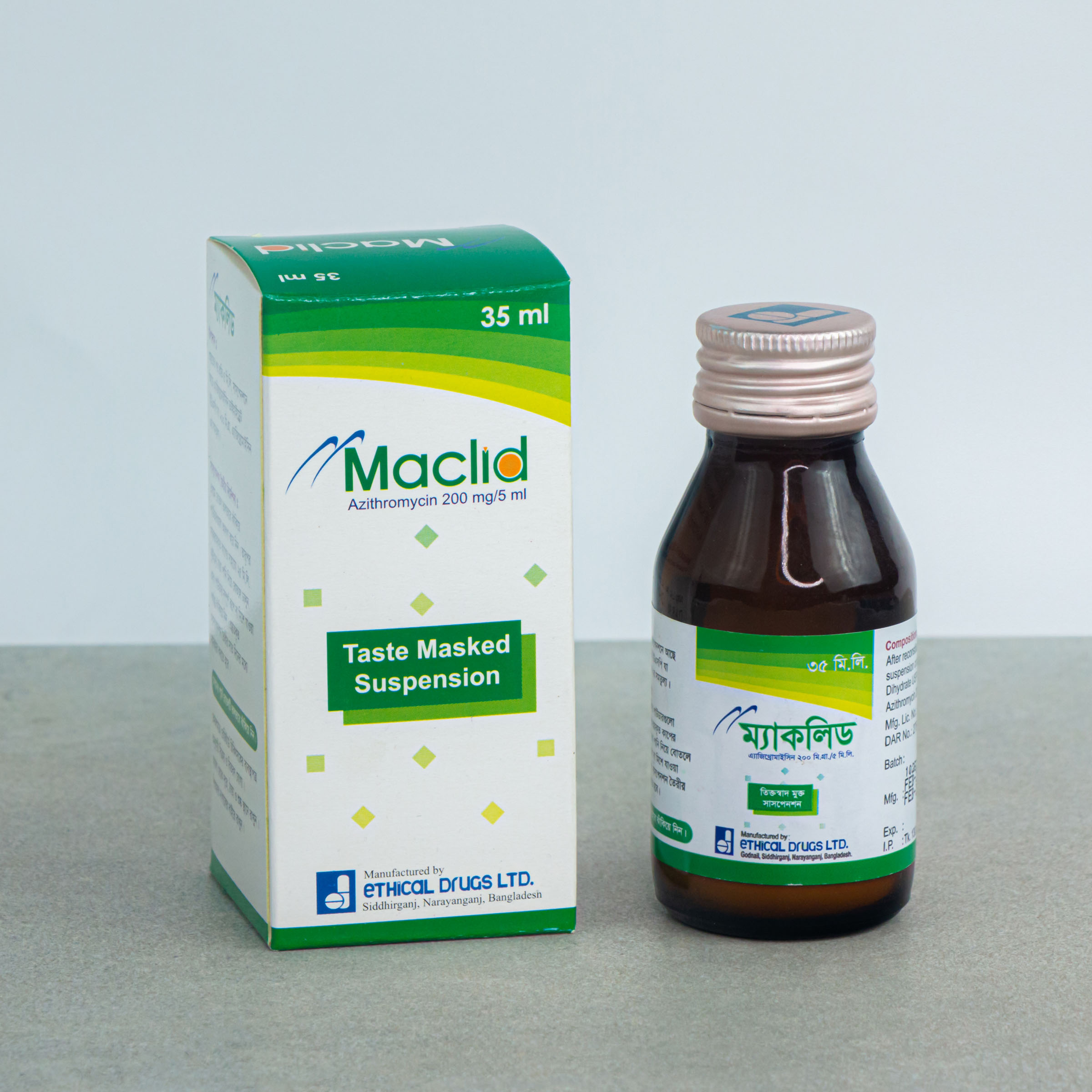
35 ml Powder for Suspension: Each Bottle contain 200 mg of Azithromycin.
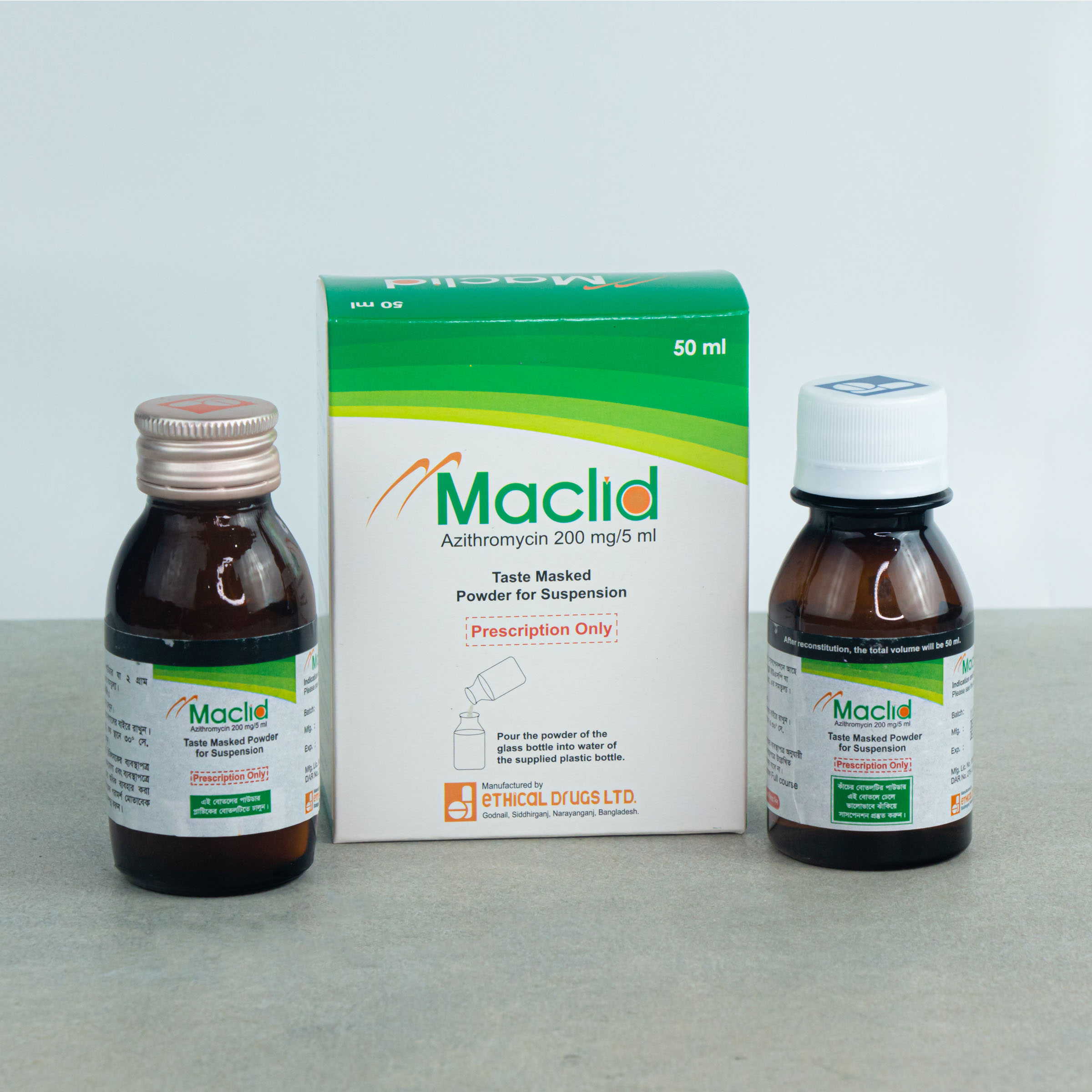
50 ml Powder for Suspension: Each Bottle contain 200 mg of Azithromycin.
PHARMACOLOGY
Azithromycin binds to the 23S rRNA of the bacterial 50S ribosomal subunit. It blocks protein synthesis by inhibiting the transpeptidation/translocation step of protein synthesis and by inhibiting the assembly of the 50S ribosomal subunit. Azithromycin concentrates in phagocytes and fibroblasts as demonstrated by in vitro incubation techniques. The ratio of intracellular to extracellular concentration was >30 after one hour incubation. In vivo studies suggest that concentration in phagocytes may contribute to drug distribution to inflamed tissues.
INDICATION
Maclid® is indicated in lower respiratory tract infections including bronchitis and pneumonia, otitis media and in upper respiratory tract infections including sinusitis and pharyngitis, tonsillitis, skin and soft tissue infections, mild to moderate typhoid due to multiple-antibacterial resistant organisms, cholera in children & adult and sexually transmitted diseases both in men and women. Azithromycin is also indicated for the treatment of uncomplicated genital infections due to Chlamydia trachomatis and urethritis and cervicitis due to Chlamydia trachomatis and Neisseria gonorrhea.
DOSGAE AND ADMINISTRATION
Maclid® capsule/tablet should be taken at least 1 hour before or 2 hours after meal. Maclid® 500 mg tablet can be taken with or without food.
Adults: For lower respiratory tract infections including bronchitis and pneumonia, upper respiratory tract infections including sinusitis and pharyngitis/tonsillitis, otitis media and skin and soft tissue infections, the total dose of Maclid® is 1.5 gm given as 500 mg once daily for 3 days.
An alternative to this dosage schedule is that 500 mg once daily on day 1, followed by 250 mg once daily for next 4 days. For sexually transmitted diseases caused by Chlamydia trachomatis, the dose of Maclid® is 1 gm given as a single dose. Alternatively, 500 mg once daily on day 1, followed by 250 mg once daily for next 2 days may also be given.
Maclid® dry syrup should be taken at least 1 hour before or 2 hours after meal. Maclid® dry syrup, add 10 ml or 2 spoonful of just boiled and cooled water to the content of the bottle and shake well to mix uniformly. There is no information on use of Azithromycin on children under 6 months of age. For children with body weight 15-25 kg (3-7 years), the dose is 200 mg once daily for 3 days, for body weight 26-35 kg (8-11 years), the dose is 300 mg once daily for 3 days, for body weight 36-45 kg (12-14 years) and the dose is 400 mg once daily for 3 days. For body weights over 45 kg, normal adult dosage is recommended.
SIDE EFFECT
Mild to moderate nausea, vomiting, abdominal pain, dyspepsia, flatulence, diarrhea, cramping, angioedema, cholestatic jaundice, dizziness, headache, vertigo, somnolence, transient elevations of liver enzyme values.
PRECAUTION
Ensure patient being treated for sexually transmitted urethritis or cervicitis has serologic test for syphilis and cultures for gonorrhea performed at time of diagnosis and that appropriate antimicrobial therapy and follow-up tests are initiated if infection is confirmed. Special Precautions: Impaired liver and renal function, pregnancy and lactation; children.
CONTRAINDICATION
Azithromycin is contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide antibiotic. Azithromycin is contraindicated in patients with a history of cholestatic jaundice and hepatic dysfunction associated with prior use of azithromycin.
DRUG INTERACTION
Antacids containing aluminium and magnesium salts reduce rate of absorption. In patients receiving Azithromycin and antacid, Azithromycin should be taken at least 1 hour before or 2 hours after antacid. Food interactions: Food may alter absorption. There have been no pharmacokinetic drug interactions between Azithromycin and warfarin, theophylline, carbamazepine, methylprednisolone and cimetidine.
USE IN PREGNANCY AND LACTATION
Azithromycin has been assigned to pregnancy category B by the FDA. Animal studies failed to reveal evidence of fetotoxicity. There are no controlled data in human pregnancy. Azithromycin should only be given during pregnancy when benefit outweighs risk. Azithromycin is excreted into human milk. The manufacturer recommends that caution be used when administering azithromycin to nursing women. In one lactating woman receiving azithromycin 500 mg/day, azithromycin milk concentration measured 1.3 and 2.8 mcg/mL 1 hour after the first dose and 30 hours after the third dose, respectively.
STORAGE
Store tablet and capsule at 30’C and Powder for Suspension at or below 25’C. Protect from light & moisture. Keep out of the reach of children
COMMERCIAL PACKAGING
MACLID®: 500 Capsule: Each box contains 3 Alu-Alu blister strips of 3 capsules.
MACLID®: 250 Capsule: Each box contains 2 Alu-Alu blister strips of 6 capsules.
MACLID®: 500 Tablet: Each box contains 1 Alu-Alu blister strip of 6 tablets.
MACLID®: Powder for Suspension 15 ml: Each bottle contains 200 mg of Azithromycin that when reconstituted with 15 ml of boiled and cooled water makes suspension of azithromycin of 40 mg/ml.
MACLID®: Powder for Suspension 35 ml: Each bottle contains 200 mg of Azithromycin that when reconstituted with 35 ml of boiled and cooled water makes suspension of azithromycin of 40 mg/ml.
MACLID®: Powder for Suspension 50 ml: Each bottle contains 200 mg of Azithromycin that when reconstituted with 50 ml of boiled and cooled water makes suspension of azithromycin of 40 mg/ml.