EFLOXIN ® – Ciprofloxacin USP
COMPOSITION
Available in 2 different Dosage forms:
Tablet: Each tablet contains 500 mg of Ciprofloxacin Hydrochloride.
Powder for Suspension: Each 5 ml contains 250 mg of Ciprofloxacin Hydrochloride.
PHARMACOLOGY
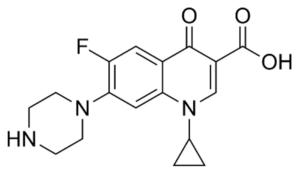
Ciprofloxacin is a synthetic 4-quinolone derivative with bactericidal activity against a wide range of gram-positive and gram-negative organism. It is active against most gram-negative aerobic bacteria including Enterobacteriaceae and Pseudomonas aeruginosa. Ciprofloxacin is also active against gram-positive aerobic bacteria including penicillinase producing, non-penicillinase producing and methicillin resistant Staphylococci. However, many strains of Streptococci are relatively resistant to the drug. The bactericidal activity of Ciprofloxacin results from interference with the enzyme DNA gyrase needed for the synthesis of bacterial DNA. The mode of action of Ciprofloxacin is different from other antibiotics like penicillin, cephalosporins, aminoglycosides, tetracyclines and for this reason it is observed that organisms resistant to these antibiotics are susceptible to Ciprofloxacin. Ciprofloxacin is well absorbed from the GIT after oral administration and it is widely distributed into the body tissues and fluid. The half-life of Ciprofloxacin is 3.5 – 4.5 hours. About 30-50% of an oral dose of Ciprofloxacin is excreted in the urine within 24 hours as unchanged drug and active metabolites.
INDICATION
Ciprofloxacin is indicated for the treatment of the following infections caused by sensitive bacteria:
Severe systemic infections: e.g.; septicaemia, bacteraemia, peritonitis, infections in immunosuppressed patients with haematological or solid tumours and in patients in intensive care unit with specific problems such as infected burns.
Respiratory tract infections: Lobar and broncho pneumonia, acute and chronic bronchitis and empyema.
Urinary tract infections: Uncomplicated and complicated urethritis, cystitis, pyelonephritis, prostatitis and epididymitis.
Skin and soft tissue infections: Infected ulcers, wound infections, abscesses, cellulitis, otitis externa, erysipelas and infected burns.
Gastrointestinal infections: Enteric fever, infective diarrhoea.
Infections of the biliary tract: Cholangitis, cholecystitis, empyema of the gall bladder.
Intra-abdominal infections: Peritonitis, intra abdominal abscesses.
Bone and joint infections: Osteomyelitis, septic arthritis.
Pelvic infections: Salpingitis, endometritis, pelvic inflammatory diseases.
Eye, ear, nose and throat infections: Otitis media, sinusitis, mastoiditis, tonsillitis.
Gonorrhoea: Urethral, rectal and pharyngeal gonorrhoea caused by beta-lactamase producing organism or organisms moderately sensitive to penicillin.
DOSAGE AND ADMINISTRATION
Adults: The usual dosage range for adults is 250-750mg twice daily.
| Type of Infection | Type of Severity | Unit Dose | Frequency | Usual Duration |
| UTI | Acute Uncomplicated | 100 mg or 250 mg | b.i.d | 3 Days |
| Mild/Moderate | 250 mg | b.i.d | 7-14 Days | |
| Severe/Complicated | 500 mg | b.i.d | 7-14 Days | |
| Lower RTI | Mild/Moderate | 500 mg | b.i.d | 7-14 Days |
| Severe/Complicated | 750 mg | b.i.d | 7-14 Days | |
| Acute Sinusitis | Mild/Moderate | 500 mg | b.i.d | 10 Days |
| SSTI | Mild/Moderate | 500 mg | b.i.d | 7-14 Days |
| Severe/Complicated | 750 mg | b.i.d | 7-14 Days | |
| Bone & Joint Infection | Mild/Moderate | 500 mg | b.i.d | ≥4-6 Weeks |
| Severe/Complicated | 750 mg | b.i.d | ≥4-6 Weeks | |
| Infectious Diarrhea | Mild/Moderate/ Severe | 500 mg | b.i.d | 3-5 Days |
| Typhoid Fever | Mild/Moderate | 500 mg | b.i.d | 10-14 Days |
| Severe | 750 mg | b.i.d | 10-14 Days | |
| Carrier Stage | 500 mg | b.i.d | 28 Days | |
| Gonococcal Infections | Uncomplicated | 250 | Single Dose | Single Dose |
Generally, treatment should be continued for 3 days after the signs and symptoms of the infection have disappeared.
Children:
Paediatric patients & adolescents (1 to 17 years of age):
Enteric fever: 10-30mg /kg/day in two divided doses for 10 days.
Acute invasive diarrhoea: 10-30mg/kg/day in two divided doses for 3 days.
Shigellosis: 10-30mg/kg/day in two divided doses for 5 days.
UTI: 20-40mg/kg/day in two divided doses for 10-21 days.
Impaired renal function: In case of severe renal impairment the total daily dose should be half.
Elderly: Dose adjustment not required.
INFORMATION FOR PATIENTS
Ciprofloxacin may be taken with or without meals and to drink fluids liberally. Concurrent administration of ciprofloxacin should be avoided with magnesium / aluminium antacids, or sucralfate or with other products containing calcium, iron or zinc. These products may be taken two hours after or six hours before ciprofloxacin.
Ciprofloxacin should not be taken concurrently with milk or yogurt alone, since absorption of ciprofloxacin maybe significantly reduced. Dietary calcium is a part of a meal, however, does not significantly affect the ciprofloxacin absorption.
SIDE EFFECT
Ciprofloxacin is generally well tolerated. Frequent adverse reactions are- Gastrointestinal disturbance: e.g., nausea diarrhoea, vomiting, dyspepsia, abdominal pain. Disturbance of the CNS: e.g., dizziness, headache, tiredness, confusion, convulsions. Hypersensitivity reactions: e.g., skin rashes, pruritus, and possible systemic reactions. Other possible side effects are – joint pain, light sensitivity, transient increase in liver enzyme (especially in patients with history of liver damage), serum bilirubin, urea or serum creatinine. Arthralgia and myalgia may also occur.
PRECAUTION
Ciprofloxacin should be used with caution in patients with a history of convulsive disorders. Crystalluria related to the use of Ciprofloxacin has been observed only rarely. Patients receiving Ciprofloxacin should be well hydrated to avoid excessive alkalinity of the urine.
CONTRAINDICATION
Ciprofloxacin is contraindicated in patients who have hypersensitivity to Ciprofloxacin or other quinolones.
DRUG INTERATIONS
Concurrent administration of Ciprofloxacin with theophylline may lead to elevated plasma concentrations of theophylline and prolongation of its elimination half-life. This may result in increased risk of theophylline related adverse reactions. If concomitant use cannot be avoided, plasma levels of theophylline should be monitored and dosage adjustments made as appropriate. Antacids containing magnesium hydroxide or aluminium hydroxide may interfere with the absorption of Ciprofloxacin resulting in serum and urine levels lower than desired, concurrent administration of these agents with Ciprofloxacin should be avoided. Probenecid interferes with renal tubular secretion of Ciprofloxacin and produces an increase in the level of Ciprofloxacin in the serum. This should be considered if patients are receiving both drugs concomitantly. As with other broad spectrum antibiotics prolonged use of Ciprofloxacin may result in overgrowth of no susceptible organism. Repeated evaluation of the patient’s condition and microbial susceptibility testing is essential. If superinfection occurs during therapy, appropriate measures should be taken.
USE IN PREGNANCY AND LACTATION
Reproduction studies performed in mice, rats and rabbits using parenteral and oral administration did not reveal any evidence of teratogenicity, impairment of fertility or impairment of pre/post natal development. However as with other quinolones, Ciprofloxacin has been shown to cause arthropathy in immature animals and therefore its use during pregnancy is not recommended. Studies in rats have indicated that Ciprofloxacin is secreted in milk, administration to nursing mothers is thus not recommended.
STORAGE
Store below 30o C in a cool and dry place, protected from light. Keep all medicines out of reach of children.
PACKAGING
EFLOXIN®: Tablets. 500 mg: Each box contains 3 strips of 10 tablets in blister pack.
EFLOXIN®: Powder for Suspension: Each bottle contains 60 ml of Ciprofloxacin Hydrochloride.