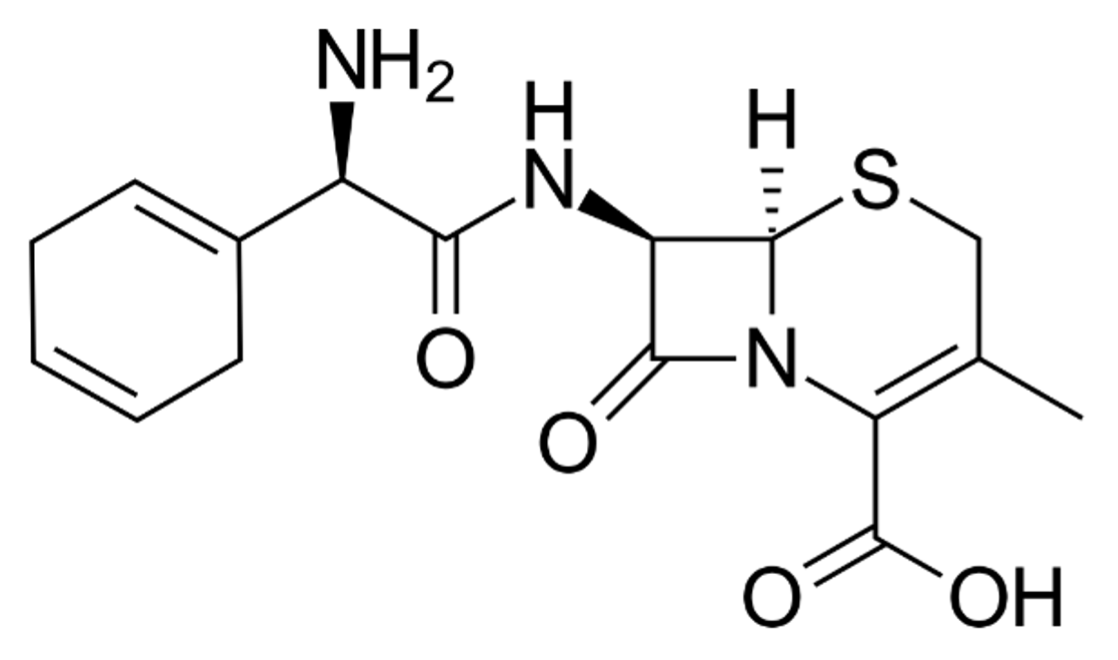
ELOCEF® – Cephradine BP
COMPOSITION
Available in 2 different Dosage forms:
250 mg Capsule: Each capsule contains Cephradine BP 250 mg.
500 mg Capsule: Each capsule contains Cephradine BP 500 mg.
Powder for Suspension: After reconstitution, each 5 ml suspension contains Cephradine BP 125 mg.
PHARMACOLOGY
Cephradine, which is the most stable first-generation Cephalosporin antibiotic, is active against all types of beta-lactamases producing bacteria. Cephradine is a broad spectrum bactericidal antibiotic active against a wide range of infections caused by susceptible Gram-negative and Gram-positive organisms. Cephradine interferes with the synthesis of bacterial cell wall by inhibiting transpeptidase enzyme. As a result, the bacterial cell wall is weakened, the cell swells and then ruptures.
INDICATION
Cephradine is indicated for the treatment of the following bacterial infections Respiratory tract infections: Tonsillitis, pharyngitis, otitis media, sinusitis, acute and chronic bronchitis, lobar and bronchopneumonia.
Skin and soft tissue infections: Cellulitis, abscess, furunculosis and impetigo. Urinary tract infections: Cystitis, urethritis, pyelonephritis and prostatitis caused by E. Coli, Proteus mirabilis, and Klebsiella species. Cephradine is also used in the treatment of some Enterococci (S. faecalis) infections confined to the urinary tract.
Gastro-lntestinal tract infections: Bacillary dysentery, enterititis & peritonitis.
Cephradine may reduce the incidence of certain postoperative infections in patients undergoing surgical procedures including dental procedure.
DOSAGE AND ADMINISTRATION
Adults: The usual dose range is 1-2 g daily in two or four equally divided doses. Severe or chronic infections may require large doses up to 4 g daily in divided doses or as advised by the physician.
Direction for reconstitution of suspension:
Shake the bottle well to losen the powder. Add 70 ml (with the help of supplied measuring cup) of boiled and cooled water to the dry mixture in the bottle. Shake the bottle vigorously until all the powder dissolve properly.
Respiratory tract infections (other than lobar pneumonia): 250 mg, 6 hourly or 500 mg, 12 hourly.
Lobar pneumonia: 500 mg, 6 hourly or 1 g, 12 hourly.
In dental procedure: 2 g orally 1 hour before the procedure.
Skin & soft tissue infections: 250 mg, 6 hourly or 500 mg, 12 hourly.
Urinary tract infections: 500 mg, 6 hourly or 1 g, 12 hourly. Severe or chronic infections may require larger doses (up to 1 g, 6 hourly).
Peritonitis: 500 mg 4 times daily.
GIT infections: 500 mg 3-4 times daily.
Prophylaxis of wound infections: 1-2 g pre-operatively, post-operative dose is adjusted as required.
Children: For children over 9 months, the usual dose is 25-50 mg/kg body weight per day, in equally divided doses, 6 or 12 hourly. For otitis media due to H. influenzae,
75-100 mg/kg body weight per day in equally divided doses, 6 or 12 hourly is recommended.
SIDE EFFECT
Adverse reactions reports are rare but include glossitis, heart burn, dizziness, nausea, vomiting, diarrhea, abdominal pain, vaginitis, candida overgrowth. Skin and hypersensitivity reactions include urticaria, skin rashes and edema. As like other cephalosporins mild transient eosinophilia, leukopenia, neutropenia and pseudomembranous colitis may be reported.
PRECAUTION
Administer cautiously to penicillin-sensitive patient because there were evidences of partial cross-allergenicity between the penicillins and cephalosporins. A modified dosage schedule in patients with impaired renal function is necessary. In patients not on dialysis, the following initial dosage should be as a guideline based on creatinine clearance (Ccr ml/min): 500 mg 6 hourly when Ccr is >20, 250 mg 6 hourly when Ccr is 5-20 & 250 mg 12 hourly when Ccr is <5. For patients on chronic, intermittent hemodialysis 250 mg initially, & repeated at 12 hours and after 36-48 hours.
Combinations with aminoglycosides may exert more potent bactericidal action against some organisms than agent alone.
Overdose: Overdose symptoms may include nausea, vomiting, stomach pain or diarrhea.
CONTRAINDICATION
Cephradine is contraindicated in patients with known hypersensitivity to cephalosporin antibiotics.
DRUG INTERACTION
The cephalosporins are potentially nephrotoxic (particularly cephaloridine) and may enhance the nephrotoxicity of aminoglycoside antibiotics such as gentamicin and tobramycin. The nephrotoxicity of cephaloridine is increased by furosemide and one should be cautious about the use of any cephalosporin with furosemide and ethacrynic acid.
Combinations with aminoglycosides may exert more potent bactericidal action against some organisms than agent alone.
USE IN PREGNANCY AND LACTATION
Animal studies with Cephradine have shown no teratogenicity. But the safety in pregnancy has not been established. Cephradine is excreted in breast milk and should be used with caution in lactating mothers.
STORAGE
Store tablet and powder for suspension at or below 30°C. Protect from light & moisture. Keep out of the reach of children. The reconstituted suspension should be used within 7 days of preparation.
PACKAGING
ELOCEF®: Capsule 25O mg: Each box contains 6 strips of 7 capsules in blister pack.
ELOCEF®: Capsule 50O mg: Each box contains 6 strips of 7 capsules in blister pack.
ELOCEF®: Powder for Suspension: Each bottle contains powder for the reconstitution of 100 ml suspension.