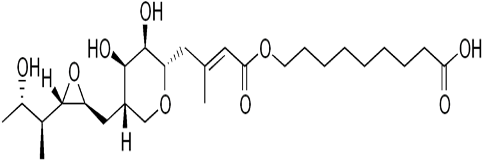
EMCIN® – Mupirocin USP
COMPOSITION
EMCIN® 2% ointment: Each gram of ointment contains Mupirocin USP 20 mg.
PHARMACOLOGY
EMCIN® is a naturally occurring antibiotic. It is an antibacterial agent produced by fermentation of Pseudomonas fluorescens. It is active against a wide range of bacteria which are responsible for the majority of skin infections, e.g. Staphylococcus aureus including methicillin-resistant Staphylococcus aureus (MRSA) other Staphylococci and Streptococci. It is also active against certain gram-negative pathogens like, E.coli and H. influenzae. EMCIN® inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl transfer-RNA synthetase. Due to this unique mode of action, EMCIN® demonstrates no in vitro cross-resistance with other classes of antimicrobial agents. Mupirocin is bactericidal at concentrations achieved by topical administration. However, the minimum bactericidal concentration (MBC) against relevant pathogens is generally eight-fold to thirty-fold higher than the minimum inhibitory concentration (MIC).
INDICATION
EMCIN® ointment is indicated for the topical treatment of impetigo due to Staphylococcus aureus and Streptococcus Pyogenes. It is also indicated in folliculitis and furunculosis.
DOSAGE AND ADMINISTRATION
A small amount of EMCIN® ointment should be applied to the affected area three times daily. The treated area may be covered with dressing gauze if desired. Patients not showing a clinical response within 3 to 5 days should be re-evaluated.
SIDE EFFECT
The following local adverse reactions have been reported in connection with the use of EMCIN® ointment: burning, stinging or pain in 1.5% of patients; itching in 1% of patients; rash, nausea, erythema, dry skin, tenderness, swelling, contact dermatitis and increased exudates in less than 1% of patients.
PRECAUTION
If chemical irritations occur with the use of EMCIN® ointment, treatment should be discontinued and appropriate alternative therapy for the infection should be maintained. As with other antibacterial products, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. In common with other polyethylene glycol-based ointments, EMCIN® should not be used; if there is evidence of moderate or severe renal impairment.
CONTRAINDICATION
This drug is contraindicated in individuals with a history of sensitivity reactions to EMCIN® or any of the components of the preparation.
DRUG INTERACTION
The effect of the concurrent application of EMCIN® and other drug products has not been studied.
USE IN PREGNANCY AND LACTATION
Use In Pregnancy: EMCIN® is classified in Pregnancy Category “B”. This drug can be used in pregnancy only if clearly needed.
Use in lactation: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when EMCIN® ointment is administered to a nursing mother.
Pediatric Use: The safety and effectiveness of EMCIN® ointment have been established in the age range of 2 months to 16 years. Use of the ointment in these age groups is supported by evidence from adequate and well-controlled studies of EMCIN® in impetigo in pediatric patients.
STORAGE
Store at or below 30° C. Do not freeze. Keep out of the reach of children.
PACKING
EMCIN® 2% ointment: Each tube contains 10gm ointment.