EURIX ® – Cefuroxime USP
COMPOSITION
Available in 2 different Dosage forms:
250 mg Tablet: Each film coated tablet contains Cefuroxime Axetil BP equivalent to Cefuroxime 250 mg.
500 mg Tablet: Each film coated tablet contains Cefuroxime Axetil BP equivalent to Cefuroxime 500 mg.
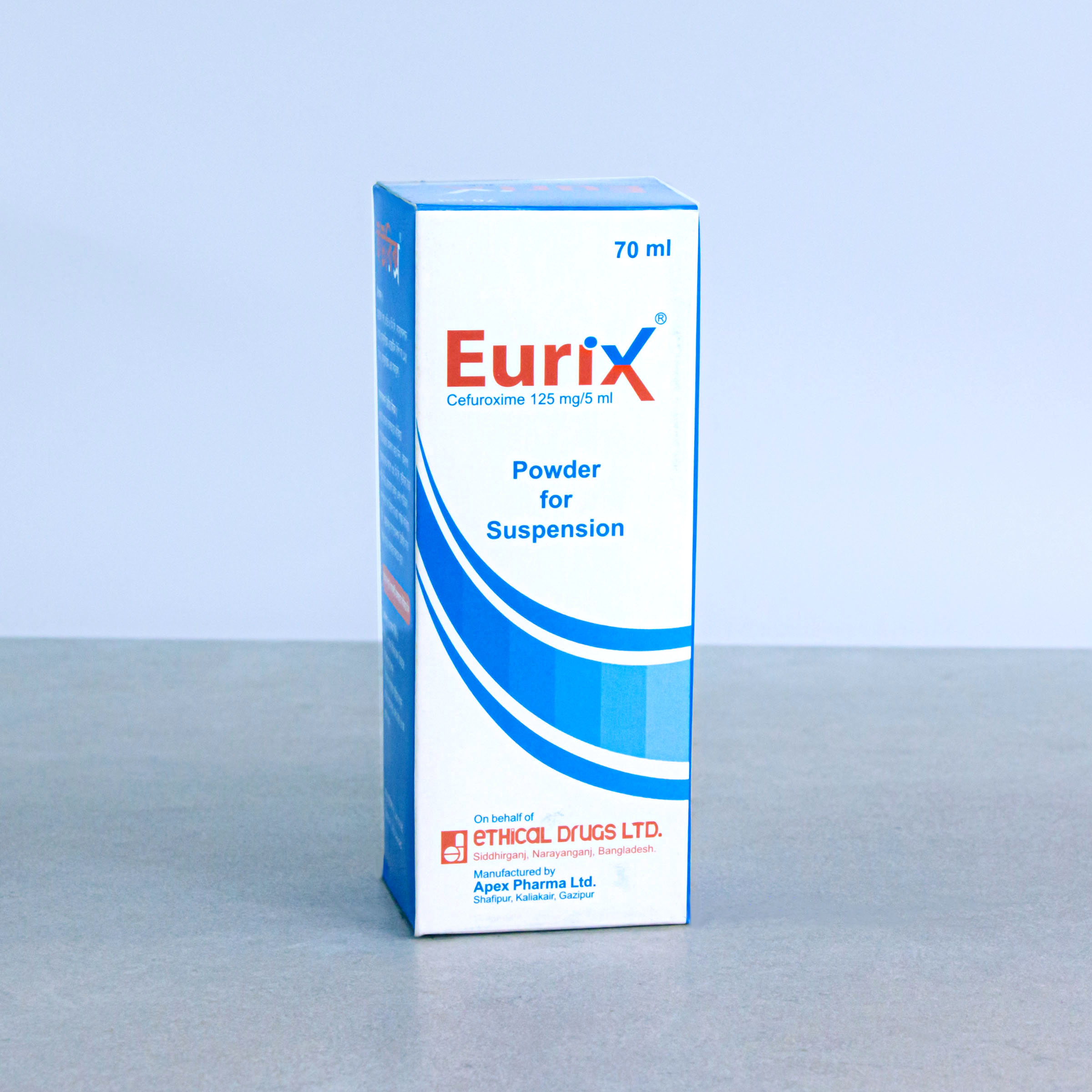
Powder for Suspension: After reconstitution, each 5 ml suspension contains Cefuroxime Axetil BP equivalent to Cefuroxime 125 mg.
PHARMACOLOGY
Cefuroxime Axetil is a broad-spectrum second-generation Cephalosporin active against a wide range of Gram-positive and Gram-negative susceptible organisms including many beta-lactamase producing strains. The bactericidal action of cefuroxime axetil results from inhibition of cell wall synthesis by binding to essential target proteins. Cefuroxime has good stability to bacterial beta-lactamases.
INDICATION
Cefuroxime is indicated in the treatment of:
Upper respiratory tract infections: Ear, nose, throat infections such as otitis media, sinusitis, tonsillitis & pharyngitis.
Lower respiratory tract infections: Acute & chronic bronchitis & pneumonia. Genitourinary tract infections: Pyelonephritis, cystitis & urethritis. gonorrhea, acute uncomplicated gonococcal urethritis & cervicitis.
Skin & tissue infections: Furunculosis, pyoderma & Impetigo.
Lyme disease: Erythema marginatum.
DOSAGE AND ADMINISTRATION
| Infections | Dosage | Duration |
| Tablet
(May be administered without regard to meals) Adolescents & adults (13 years & above) Pharyngitis of tonsillitis Paediatric patients (upto 12 years) (Who can swallow tablets whole) Pharyngitis or tonsilitis |
250 mg twice daily 125 mg twice daily |
5-10 days 5-10 days |
| Suspension
(Must be administered immediately after meal. Shake the bottle well before each use) Paediatric patients (3 months to 12 years) |
20 mg/kg/day in two divided doses |
5-10 days |
SIDE EFFECT
Generally, Cefuroxime is well tolerated. However, a few side effects like nausea, vomiting, diarrhea, abdominal pain may occur. As with other broad spectrum antibiotics prolonged administration of Cefuroxime Axetil may result in overgrowth of nonsusceptible microorganisms. Rarely renal dysfunction, pruritus, angioedema, rash and serum sickness like urticaria may appear.
PRECAUTION
Cefuroxime should be given with care to patients receiving concurrent treatment with potent diuretics & who have history of colitis.
CONTRAINDICATION
Contraindicated in patients with known allergy to cephalosporin & having pseudomembranous colitis.
DRUG INTERACTION
Concomitant administration of probenecid with Cefuroxime increases the area under the serum concentration versus time curve by 50%. Drug that reduces gastric acidity may result in a lower bioavailability of Cefuroxime and tend to cancel the effect of postprandial absorption.
Over Dosage:
Signs and symptoms: Overdose of Cefuroxime can cause cerebral irritation leading to convulsions.
Management: Serum levels of Cefuroxime can be reduced by hemodialysis and peritoneal dialysis.
Direction for reconstitution of suspension:
Shake the bottle well to loosen the powder. Add 70 ml (with the help of supplied measuring cup) of boiled and cooled water to the dry mixture in the bottle. Shake the bottle vigorously until all the powder dissolve properly.
USE IN PREGNANCY AND LACTATION
Pregnancy: Pregnancy category ‘B’. While all antibiotics should be avoided in the first trimester if possible. However, Cefuroxime has been safely used in later pregnancy to treat urinary infections.
Nursing mother: Cefuroxime is excreted into the breast milk in small quantities. However, the possibility of sensitizing the infant should be kept in mind.
STORAGE
Store tablet and Powder for Suspension at or below 30°C. Protect from light & moisture. Keep out of the reach of children.
PACKAGING
EURIX®: 250 mg Tablet: Each box contains 3 Strips of 7 tablets in Alu-Alu blister pack.
EURIX®: 500 mg Tablet: Each box contains 2 Strips of 7 tablets in Alu-Alu blister pack.
EURIX®: Powder for Suspension: Each bottle contains powder for reconstitution of 70 ml suspension.