FLUNID® – Flucloxacillin Sodium BP
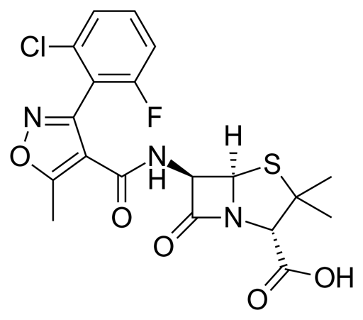
COMPOSITION
Available in 2 different Dosage forms:
250 mg Capsule: Each capsule contains Flucloxacillin Sodium BP equivalent to Flucloxacillin 250 mg.
500 mg Capsule: Each capsule contains Flucloxacillin Sodium BP equivalent to Flucloxacillin 500 mg.
Powder for Suspension: After reconstitution, each 5 ml of suspension contains Flucloxacillin Sodium BP equivalent to Flucloxacillin 125 mg.
PHARMACOLOGY
Flucloxacillin is isoxazolyl penicillin which combine the properties of resistance to hydrolysis by penicillinase, gastric acid stability and activity against gram-positive bacteria. Flucloxacillin is a bactericidal antibiotic that is particularly useful against penicillinase-producing staphylococci.
INDICATION
Flucloxacillin is indicated for the treatment of infections due to gram positive organisms, including infections caused by -lactamase producing staphylococci.
Skin and soft tissue infections: boils, abscesses, carbuncles, furunculosis, cellulitis; infected skin conditions, e.g., ulcer, eczema and acne; infected wounds, infected burns, protection for skin grafts, otitis media and externa, impetigo.
Respiratory tract infections: pneumonia, lung abscess, empyema, sinusitis, pharyngitis, tonsillitis, and quinsy.
Other infections caused by Flucloxacillin-sensitive organisms: osteomyelitis, enteritis, endocarditis, urinary tract infections, meningitis, septicemia.
Flucloxacillin is also indicated for use as a prophylactic agent during major surgical procedures where appropriate ; for example, cardiothoracic and orthopedic surgery.
DOSAGE AND ADMINISTRATION
Oral doses should be administered half to one hour before meals.
Usual adult dose (including elderly patients): 250 mg four times daily. In case of severe infections, dosage should be doubled.
In osteomyelitis and endocarditis: Up to 8 gm daily, in divided doses 6 to 8 hourly. In case of secondary bacterial infection-in chicken pox: Flucloxacillin 500 mg 6 hourly should be prescribed.
Usual children dose:
2-10 years: Half of the adult dose.
Under 2 years: Quarter of the adult dose.
SIDE EFFECT
Side effects as with other penicillin, are uncommon and mainly of a mild and transitory nature. Gastro-intestinal upsets (e.g., nausea, diarrhea) and skin rashes have been reported. If skin rash occurs, treatment should be discontinued.
PRECAUTION
Flucloxacillin should be used with caution in patients with evidence of hepatic dysfunction; Caution should be exercised in the treatment of patients with an allergic diathesis.
In case of, Flucloxacillin powder for suspension, reconstituted suspension is to be consumed within 7 days of preparation.
CONTRAINDICATION
This drug is contraindicated in patient with hypersensitivity to the active ingredient or any component of the drug.
DRUG INTERACTION
The administration of probenecid with Flucloxacillin results in higher serum peak concentrations and prolongs the time that therapeutic concentrations of Flucloxacillin are achieved in serum. Physical incompatibility and/or loss of activity of Flucloxacillin in solution has been reported when given with gentamycin sulphate, streptomycin sulphate, vitamin mixtures.
Flucloxacillin should not be added to intravenous lipids, blood products and protein hydrolysates or other proteinaceous fluids.
Overdose:
Problems of overdose with Flucloxacillin are unlikely to occur; if encountered they may be treated symptomatically.
Direction for reconstitution of suspension:
At first, shake the bottle well to loosen the powder. Add 14 tea- spoonful (70 ml) of boiled cool water to the bottle and shake well. Reconstituted suspension should be consumed within one week of preparation.
USE IN PREGNANCY AND LACTATION
The use of Flucloxacillin in pregnancy should be reserved for cases considered essential by the clinician. Use of the drug in the second and third trimesters may result in the sensitization of the fetus. During lactation, trace quantities of penicillin can be detected in breast milk.
Use in Children & Adolescents:
Animal studies show that high doses of Flucloxacillin reduce albumin bound bilirubin to 50 to 70% of the base line concentration. The drug should therefore be used with extreme caution in jaundiced neonates or premature neonates.
STORAGE
Store tablet at 30°C and Powder for Suspension at or below 25°C. Protect from light & moisture. Keep out of the reach of children.
PACKAGING
FLUNID®: 250 mg Capsule: Box containing 4 Strips of 7 Capsules in Alu-Alu blister.
FLUNID®: 500 mg Capsule: Box containing 4 Strips of 7 Capsules in Alu-Alu blister.
FLUNID®: Powder of Suspension: Bottle containing powder for the preparation of 100 ml suspension.