GASPRO®–Esomeprazole BP
COMPOSITION
Available in 2 different Dosage forms:
20 mg Capsule: Each capsule contains 20 mg of Esomeprazole.
20 mg Capsule: Each capsule contains 20 mg of Esomeprazole.
40 mg IV Injection: Each box contains 1 vial of lyophilized Esomeprazole 40mg, 1 ampoule of 5 ml 0.9% sodium chloride solution BP & 1 syringe (5ml)
PHARMACOLOGY
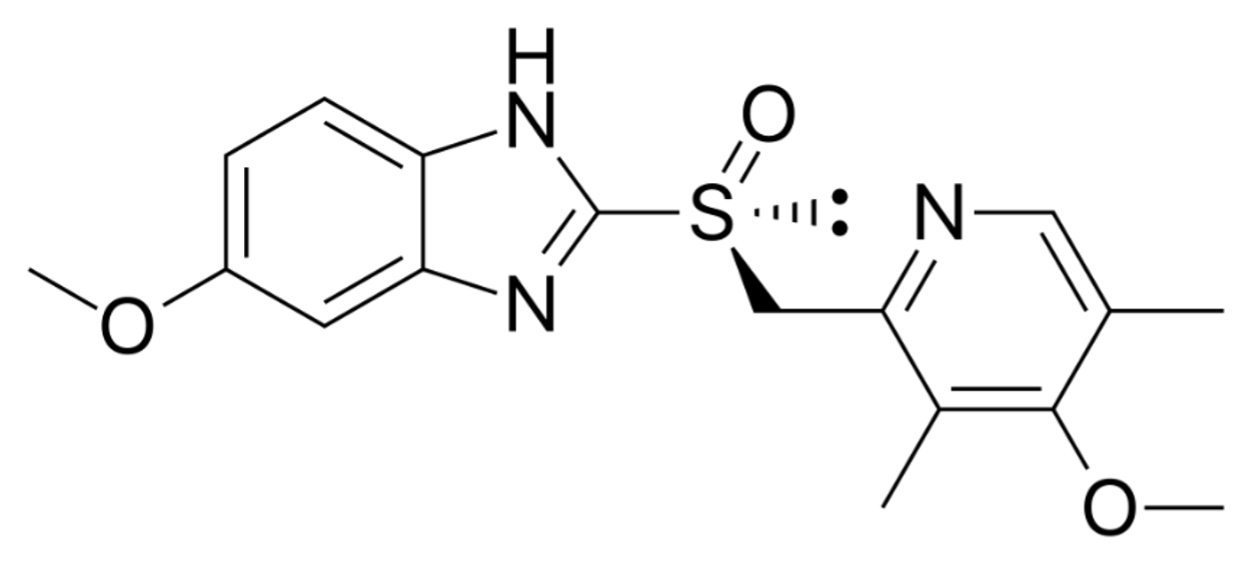
Gaspro® is a preparation of Esomeprazole which is the S-isomer of Omeprazole, a proton pump inhibitor, weak base in nature and is concentrated and converted to the active form in the highly acidic environment of the intracellular canaliculi within the parietal cell, where it inhibits the enzyme H+/K+-ATPase, the acid pump. This effect on the final step of the gastric acid formation process is dose-dependent and provides for highly effective inhibition of both basal and stimulated acid secretion, irrespective of the stimulus.
INDICATION
GASPRO® capsule is indicated for:
Gastroesophageal reflux disease (GERD), treatment of erosive reflux esophagitis, maintenance of healing of erosive esophagitis, symptomatic treatment of gastroesophageal reflux disease (GERD), eradication of Helicobacter pylori (In combination with an appropriate antibiotics), healing of Helicobacter pylori associated duodenal ulcer, prevention of relapse of peptic ulcers in patient with Helicobacter pylori associated, patients requiring continued NSAID therapy , healing of gastric ulcers associated with NSAID therapy, prevention of gastric and duodenal ulcers associated with NSAID, treatment Zollinger-Ellison syndrome , etc
DOSAGE AND ADMINISTRATION
For adults and adolescents from the age of 12 years:
Gaspro® tablet should be taken one hour before meal.
INDICATION DOSE FREQUENCY
Gastroesophageal Reflux Disease (GERD)
Healing of Erosive Esophagitis 20 mg or 40 mg Once Daily for 4 to 8 Weeks
Maintenance of Healing of Erosive Esophagitis 20 mg Once Daily
Symptomatic Gastroesophageal Reflux Disease 20 mg Once Daily for 4 Weeks
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Triple Therapy:
Gaspro® 40 mg Once Daily for 10 Days
Amoxicillin 1000 mg Twice Daily for 10 Days
Clarithromycin 500 mg Twice Daily for 10 Days
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome 40 mg Twice Daily
For Children:
In GERD (1 to 11 years) 10mg to 20mg Esomeprazole once daily for up to 8 weeks.
For Elderly:
Dosage adjustment is not required in elderly patients.
Patients with impaired hepatic function:
No dosage adjustment is necessary in patients with mild to moderate liver impairment. For patients with severe liver impairment a dose of 20 mg of Gaspro® should not be exceeded.
Patients with renal impairment:
No dose adjustment is necessary in patients with impaired renal function.
SIDE EFFECT
The most commonly reported side effects were nausea, vomiting, abdominal pain, flatulence, diarrhoea, constipation and headache. Less frequent side-effects include dry mouth, peripheral oedema, dizziness, sleep disturbances, fatigue, paraesthesia, arthralgia, myalgia, rash, and pruritus. Other side-effects reported rarely or very rarely include taste disturbance, stomatitis, hepatitis, jaundice, hypersensitivity reactions (including anaphylaxis, bronchospasm), fever, depression, hallucinations, confusion, gynaecomastia, interstitial nephritis, hyponatraemia, blood disorders (including leukopenia, leucocytosis, pancytopenia, thrombocytopenia), visual disturbances, sweating, photosensitivity, alopecia, Stevens-Johnson syndrome, and toxic epidermal necrolysis.
PRECAUTION
Symptomatic response to therapy with Esomeprazole does not preclude the presence of gastric malignancy. When gastric ulcer is suspected or present, malignancy should be excluded, as treatment with Esomeprazole may alleviate symptoms and delay diagnosis. When prescribing Esomeprazole for eradication of Helicobacter pylori possible drug interactions for all components in the triple therapy should be considered.
CONTRAINDICATION
Esomeprazole is contraindicated in patients with known hypersensitivity to any component of the formulation or to substituted benzimidazoles.
DRUG INTERACTION
Esomeprazole may interfere with the absorption of drugs where gastric pH is an important determinant of bioavailability (e.g., ketoconazole, iron salts and digoxin). Coadministration of atazanavir with Esomeprazole is expected to substantially decrease atazanavir plasma concentrations and thereby reduce its therapeutic effect. Esomeprazole inhibits CYP2C19, the major Esomeprazole metabolising enzyme. Thus, when Esomeprazole is combined with drugs metabolised by CYP2C19, such as diazepam, citalopram, imipramine, clomipramine, phenytoin etc., the plasma concentrations of these drugs may be increased and a dose reduction could be needed.
USE IN PREGNANCY AND LACTATION
Pregnancy category B drug. Esomeprazole may be used in pregnancy if clearly needed. Although safety during pregnancy has not been adequately evaluated. The excretion of esomeprazole in milk has not been studied. It is not known whether this drug is excreted in human milk. Taking into account the importance of the drug to the mother, a decision should be made whether to discontinue nursing or to discontinue the drug.
STORAGE
Store capsules at or below 30°C. Protect from light & moisture. Keep out of the reach of children. The reconstituted suspension should be used within 7 days of preparation.
PACKAGING
GASPRO® 20: Capsules 20 mg: Each box contains 5 strips of 10 capsules in blister pack.
GASPRO® 40: Capsules 40 mg: Each box contains 5 strips of 10 capsules in blister pack.
GASPRO® IV Injection: Each box contains 1 vial of lyophilized Esomeprazole 40mg, 1 ampoule of 5 ml 0.9% sodium chloride solution BP & 1 syringe (5ml).