K-LAC® – Ketorolac Tromethamine USP
COMPOSITION
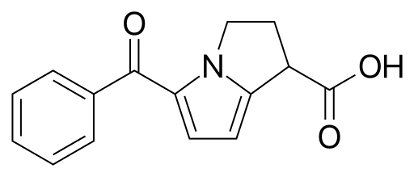
K-LAC® 10 Tablet: Each tablet contains Ketorolac Tromethamine USP 10 mg.
K-LAC® 30 IM/IV Injection: Each ml Ampoule contains Ketorolac Tromethamine USP 30 mg.
K-LAC® 60 IM/IV Injection: Each 2 ml Ampoule contains Ketorolac Tromethamine USP 60 mg.
PHARMACOLOGY
K-LAC® (Ketorolac Tromethamine) is a non-steroidal anti-inflammatory drug that exhibits analgesic activity. Ketorolac Tromethamine inhibits synthesis of prostaglandins and may be considered a peripherally acting analgesic. Ketorolac Tromethamine possesses no sedative or anxiolytic properties.
INDICATION
K-LAC® is indicated for short term management of moderate to severe acute postoperative pain and other acute pains.
DOSAGE AND ADMINISTRATION
By mouth, 10 mg every 4-6 hours (the elderly every 6-8 hours): Max 40 mg daily; Maximum duration of treatment 7 days. By intravenous / intramuscular injection over not less than 15 seconds, initially 10mg, then 10-30 mg every 4-6 hours when required (every 2 hours in initial postoperative period); Max. 90 mg. Daily (the elderly and patients wt. Less than 50 Kg. max. 60 mg daily); Max duration of treatment 2 days by either route. When converting from parenteral to oral administra¬tion, total combined dose on the day converting should not exceed 90 mg (60 mg in the elderly and patients wt. less than 0 kg) of which the oral component should not exceed 40 mg, patients should be converted to oral routs as soon as possible.
SIDE EFFECT
Anaphylaxis, dry mouth, excessive thirst, hyponatremia, hyperkalaemia, flushing or pallor, bradycardia, hypertension, palpitations, chest pain, purpura, postoperative wound haemorrhage.
PRECAUTION
K-LAC® should be used with caution in heart failure, hepatic impairment and conditions leading to reduction in blood volume or in renal blood flow. The dose of ketorolac should be low in case of the elderly and in patients wt. less than 50 kg, It is recommended that patients with mild renal impairment should receive are due dose of Ketorolac and undergo close monitoring of renal function.
CONTRAINDICATION
K-LAC® is contraindicated in patients who are hypersensitive to any component of this product. It is also contraindicated in patients with history of asthma, complete or partial syndrome of nasal polyps. angioedema or bronchospasm, history of peptic ulceration or gastrointestinal bleeding, moderate or severe renal impairment, hypovolaemia or dehydration, pregnancy (including labour and delvery) and breast-feeding.
DRUG INTERACTION
K-LAC® should not be given to patients already receiving anticoagulants, or to those who will require prophylactic anticoagulant therapy, including low dose heparin. The risk of ketorolac associated bleeding is increased by other NSAIDs or aspirin and by pentoxifylling and thus concomitant use should be avoided.
USE IN PREGNANCY AND LACTATION
K-LAC® is contraindicated in the later stages of pregnancy and should only be used earlier in the pregnancy if there are compelling reasons. Because of the possible adverse effects of prostaglandin inhibiting drugs on neonates, use in nursing mother is contraindicated.
Paediatric use:
Safety and effectiveness have not been established in paediatric patients below the ages of 17 years.
The elderly:
Dose of ketorolac should not exceed 60 mg daily in the elderly.
Paediatric use:
Safety and effectiveness have not been established in paediatric patients below the ages of 17 years.
The elderly:
Dose of ketorolac should not exceed 60 mg daily in the elderly.
STORAGE
Store in a cool, dry place & below 30°C. Protect from light & moisture. Keep out of the reach of children.
PACKAGING
Available in two dosage form
K-LAC® 10 Tablet: Each box containing 3×10’s tablets in blister pack
K-LAC® 30 IM/IV Injection: Each box containing 1m1 x1 ampoule in blister pack.
K-LAC® 60 IM/IV Injection: Each box containing 2m1 x1 ampoule in blister pack.