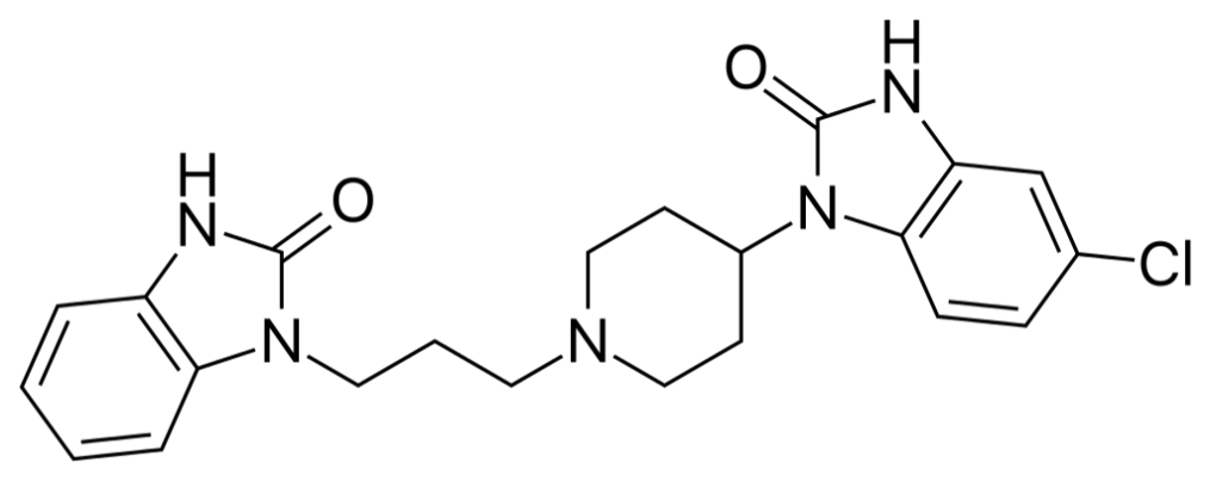
MOTILIUM ®– Domperidone Maleate BP
COMPOSITION
Available in 2 different Dosage forms:
10 mg Tablet: Each tablet contains Domperidone Maleate equivalent to Domperidone BP 10 mg.
Suspension: Each 5 ml suspension contains Domperidone Maleate equivalent Domperidone BP 5 mg.
PHARMACOLOGY
Domperidone is a dopamine-receptor blocking agent. Its action on the dopamine receptors in the chemo-emetic trigger zone produces an anti-emetic effect. It does not cross the blood-brain barrier to any appreciable degree and so exerts relatively little effect on cerebral dopaminergic receptors. Domperidone has been shown to increase the duration of antral and duodenal contractions to increase gastric emptying. It does not alter gastric secretions and has no effect on intracranial pressure or on the cardiovascular system. Domperidone is rapidly absorbed, with peak plasma concentrations at approximately 1 hour after oral administration. The plasma half-life after a single oral dose is 7 to 9 hours in healthy subjects but is prolonged in patients with severe renal insufficiency. Urinary and faecal excretion amount to 31% and 66% of the oral dose, respectively. The proportion of drug excreted unchanged is small (approximately 1% of urinary and 10% of faecal excretion).
INDICATION
1. Stimulation of gut motility in non-ulcer dyspepsia, Esophageal reflux, reflux oesophagitis and gastritis, Diabetic gastroparesis, Functional dyspepsia.
2. Prevention and symptomatic relief of acute nausea and vomiting from any cause including cytotoxic therapy, radiotherapy and anti-parkinsonism therapy.
3. In the prophylactic treatment of migraine.
DOSAGE AND ADMINISTRATION
Domperidone should be taken 15-30 minutes before meals. The usual recommended oral dose for: Adults: 10-20 mg every 4-8 hours daily. Children (2-12 years): 0.2-0.4 mg/kg body weight (0.4-0.8ml paediatric drops/10 kg) every 4-8 hours daily.
SIDE EFFECT
Allergic reactions, such as rash or urticaria, have been reported. Abdominal cramps have been reported. Unusual production of breast milk in men and women. Breast enlargement in men and women. Lowering of sexual drive (libido) in men. Menstrual periods may be irregular or stop. Problems with vision. Development of a tremor.
PRECAUTION
Domperidone should be used with caution in:
Infants. Liver disorders. Kidney disorders
CONTRAINDICATION
It should not be used in:
Allergies to domperidone or any of the other ingredients. Tumour of the pituitary gland (prolactinoma). Stomach or duodenal ulcer. Serious heart conditions. Frequent/severe stomach cramps. A blockage, tear or perforated ulcer in your intestines, breast feeding and severe liver or kidney disease.
DRUG INTERATIONS
This drug should not be used with the following medications because very serious, possibly fatal interactions may occur:
Oral ketoconazole (used for fungal infections)
Erythromycin (an antibiotic)
Telaprevir
Ritonavir (used for HIV infection)
USE IN PREGNANCY AND LACTATION
In the case of pregnancy or expected pregnancy, physician’s advice must be availed before taking this medicine.
Domperidone is not safe while breastfeeding.
STORAGE
Store tablet and suspension at or below 30°C. Protect from light & moisture.
Keep out of the reach of children.
PACKAGING
MOTILIUM®: Tablet 10 mg: Each box contains 10 strips of 10 tablets in blister pack.
MOTILIUM®: Suspension: Each bottle contains 60 ml of suspension.