NILICON® – Ceftriaxone Sodium BP
COMPOSITION
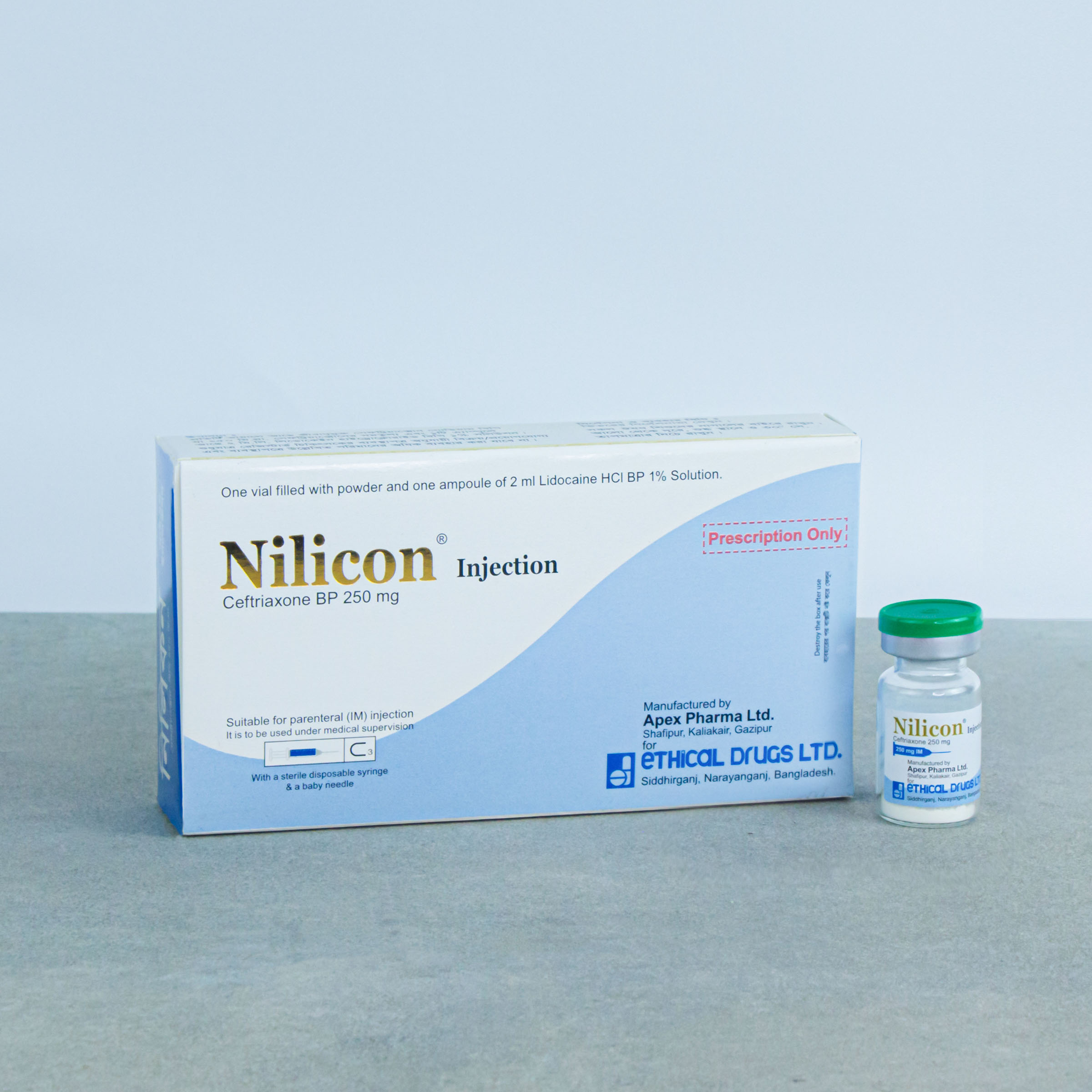
250 mg IM/IV Injection: Each vial contains sterile Ceftriaxone Sodium BP equivalent to 250 mg of Ceftriaxone and 1 ampoule of 2 ml Lidocaine Hydrochloride BP 1% solution for IM injection or 5 ml water for Injection BP for IV injection.
500 mg IM/IV Injection: Each vial contains sterile Ceftriaxone Sodium BP equivalent to 500 mg of Ceftriaxone and 1 ampoule of 2 ml Lidocaine Hydrochloride BP 1% solution for IM injection or 5 ml water for Injection BP for IV injection.
1 g IM/IV Injection: Each vial contains sterile Ceftriaxone Sodium BP equivalent to 1 g of Ceftriaxone and 1 ampoule of 3.5 ml Lidocaine Hydrochloride BP 1% solution for IM injection or 10 ml water for Injection BP for IV injection.
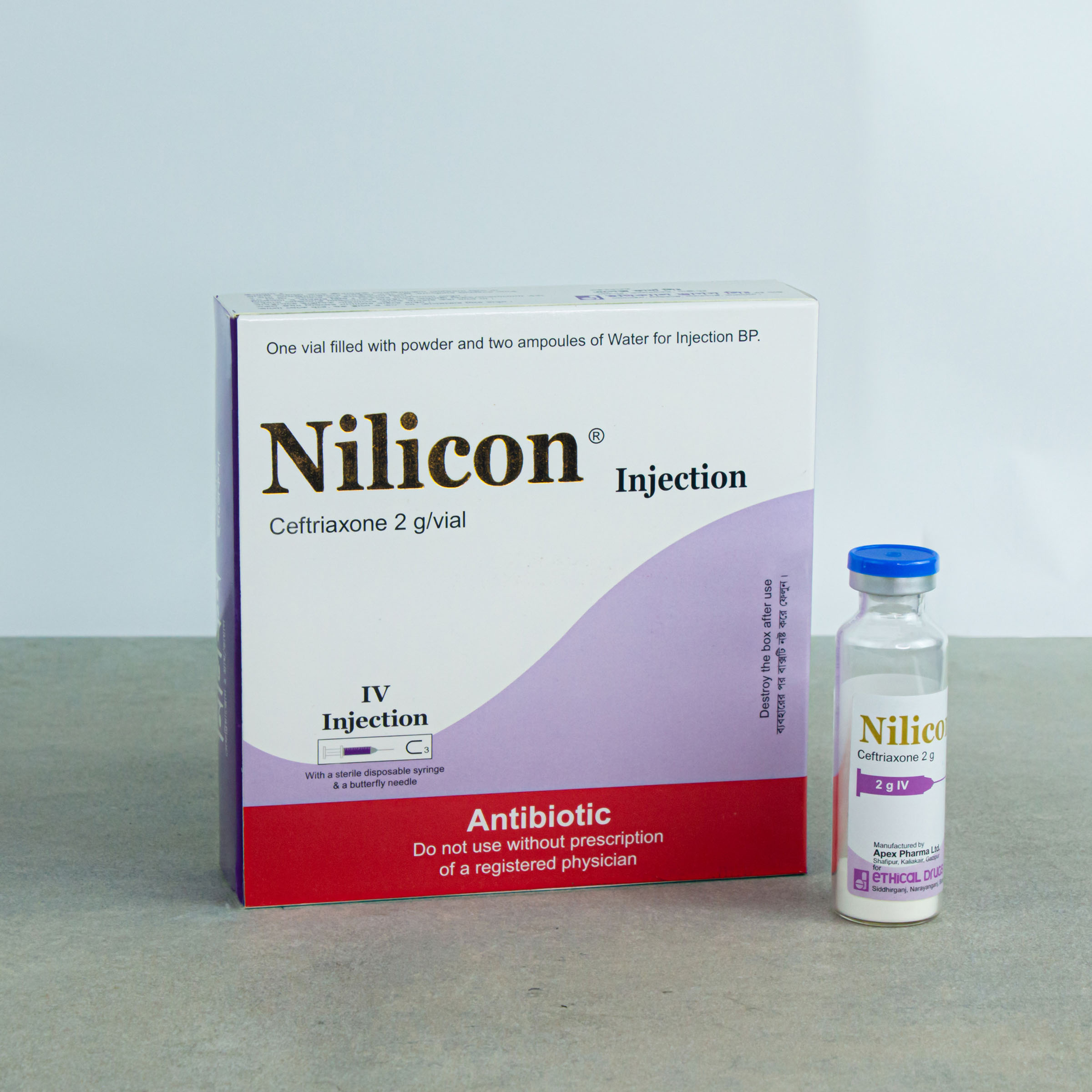
2 g IV Injection: Each vial contains sterile Ceftriaxone Sodium BP equivalent to 2 g of Ceftriaxone and 1 ampoule of 10 ml water for Injection BP for IV injection.
PHARMACOLOGY
Nilicon® is Ceftriaxone preparation. Nilicon® is a sterile, semisynthetic, broad-spectrum, 3rd generation cephalosporin antibiotic for intravenous or intramuscular administration. The bactericidal activity of Ceftriaxone results from inhibition of cell wall synthesis. Ceftriaxone has a high degree of stability in the presence of beta-lactamases both penicillinases and cephalosporinases of gram-negative and gram-positive bacteria.
INDICATION
Nilicon® is mostly utilized for prevention of post operative infections and preoperative prophylaxis of infections associated with surgery. Other than that, Nilicon® is indicated for the treatment of the following infections when caused by susceptible organisms:
Lower Respiratory Tract Infections
Acute Bacterial Otitis Media
Skin and Skin Structure Infections
Urinary Tract Infections
Uncomplicated Gonorrhea
Pelvic Inflammatory Disease
Bacterial Septicemia
Bone and Joint Infections
Intra-abdominal Infections
Meningitis
Surgical Prophylaxis
Renal infection
DOSAGE AND ADMINISTRATION
Ceftriaxone can be administered either intravenously or intramuscularly. Prior to entering/administrating the medicine to patient’s body test dose is given to examine the tolerance of the dose. Adults: The usual adult dose is 1-2 g once daily, (or twice daily in equally divided doses) depending on the type and severity of infections. In severe cases, the dose may be increased to 4g once daily. For preoperative use (surgical prophylaxis), a single dose of 1 gm administered intravenously 0.5-2 hours before surgery is recommended. In patients with impaired renal function and liver damage, there is no need to reduce the dosage. Gonorrhea: For the treatment of gonorrhea (penicillinase producing and non-penicillinase producing strains), a single intramuscular dose of 250 mg is recommended. Children under 12 years: The recommended total daily dose is 50 to 75 mg/kg once daily (or twice daily in equally divided doses). In severe infections, up to 80 mg/kg body weight daily may be given. The total daily dose should not exceed 2 g. In the treatment of meningitis, the initial dose is 100 mg/kg body weight (not to exceed 4 g daily) once daily (or twice daily in equally divided doses). The usual duration of therapy in meningitis is 7 to 14 days.
SIDE EFFECT
Ceftriaxone is generally well tolerated. However, few side-effects including diarrhea, nausea vomiting; rash, pruritus, urticaria, edema & erythema multiforme eosinophilia, thrombocytosis, leukopenia, neutropenia, elevations of SGOT or SGPT, bilirubinemia; headache, hyperactivity, nervousness, sleep disturbances, confusion, hypertonia and dizziness may occur. Local phlebitis occurs rarely following intravenous administration but can be minimized by slow injections over 2-4 minutes. Convulsion and involuntary movement may occur.
PRECAUTION
Ceftriaxone should be administered with caution to individuals with a history of gastrointestinal disease, particularly colitis.
CONTRAINDICATION
Ceftriaxone is contraindicated in patients with known allergy to ceftriaxone, other cephalosporins or penicillins.
DRUG INTERACTION
No impairment of renal function or increased nephrotoxicity has been observed after simultaneous administration of Ceftriaxone with diuretics or with aminoglycosides.
Overdose:
In the case of overdosage, drug concentration would not be reduced by hemodialysis or peritoneal dialysis. There is no specific antidote. Treatment of overdosage should be symptomatic.
Use in children:
Safety and effectiveness of Ceftriaxone in neonates, infants and pediatric patients have been established for the dosages described in the DOSAGE AND ADMINISTRATION section.
USE IN PREGNANCY AND LACTATION
Pregnancy: The safety of Ceftriaxone in the treatment of infections during pregnancy has not been established. Ceftriaxone should only be used during pregnancy if the likely benefit outweighs the potential risk to the fetus and/or the mother.
Lactation: Ceftriaxone is excreted in breast milk at low concentrations. Therefore, caution should be exercised when Ceftriaxone is administered to a nursing mother.
STORAGE
Store below 30°C and protect from light & moisture. Keep out of the reach of the children.
PACKAGING
NILICON®: 250 mg IM Injection: Combipack of 1 vial of 250 mg of Ceftriaxone (as sterile Ceftriaxone Sodium BP) and 1 ampoule of 2 ml Lidocaine Hydrochloride BP 1% solution.
NILICON®: 250 mg IV Injection: Combipack of 1 vial of 250 mg of Ceftriaxone (as sterile Ceftriaxone Sodium BP) and 1 ampoule of 5 ml Water for Injection BP.
NILICON®: 500 mg IM Injection: Combipack of 1 vial of 500 mg of Ceftriaxone (as sterile Ceftriaxone Sodium BP) and 1 ampoule of 2 ml Lidocaine Hydrochloride BP 1% solution.
NILICON®: 500 mg IV Injection: Combipack of 1 vial of 500 mg of Ceftriaxone (as sterile Ceftriaxone Sodium BP) and 1 ampoule of 5 ml Water for Injection BP.
NILICON®: 1 g IM Injection: Combipack of 1 vial of 1 g of Ceftriaxone (as sterile Ceftriaxone Sodium BP) and 1 ampoule of 3.5 ml Lidocaine Hydrochloride BP 1% solution.
NILICON®: 1 g IV Injection: Combipack of 1 vial of 1 g of Ceftriaxone (as sterile Ceftriaxone Sodium BP) and 1 ampoule of 10 ml Water for Injection BP.
NILICON®: 2 g IV Injection: Combipack of 1 vial of 2 g of Ceftriaxone (as sterile Ceftriaxone Sodium BP) and 1 ampoule of 10 ml Water for Injection BP.